Cone Genesis Tracing by the Chrnb4-EGFP Mouse Line: Evidences of Cellular Material Fusion after Cone Precursor Transplantation
- PMID: 28143742
- PMCID: PMC5363218
- DOI: 10.1016/j.ymthe.2016.12.015
Cone Genesis Tracing by the Chrnb4-EGFP Mouse Line: Evidences of Cellular Material Fusion after Cone Precursor Transplantation
Abstract
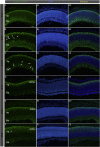
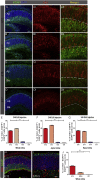
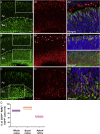
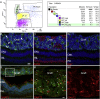
The cone function is essential to mediate high visual acuity, color vision, and daylight vision. Inherited cone dystrophies and age-related macular degeneration affect a substantial percentage of the world population. To identify and isolate the most competent cells for transplantation and integration into the retina, cone tracing during development would be an important added value. To that aim, the Chrnb4-EGFP mouse line was characterized throughout retinogenesis. It revealed a sub-population of early retinal progenitors expressing the reporter gene that is progressively restricted to mature cones during retina development. The presence of the native CHRNB4 protein was confirmed in EGFP-positive cells, and it presents a similar pattern in the human retina. Sub-retinal transplantations of distinct subpopulations of Chrnb4-EGFP-expressing cells revealed the embryonic day 15.5 high-EGFP population the most efficient cells to interact with host retinas to provoke the appearance of EGFP-positive cones in the photoreceptor layer. Importantly, transplantations into the DsRed retinas revealed material exchanges between donor and host retinas, as >80% of transplanted EGFP-positive cones also were DsRed positive. Whether this cell material fusion is of significant therapeutic advantage requires further thorough investigations. The Chrnb4-EGFP mouse line definitely opens new research perspectives in cone genesis and retina repair.
Keywords: cones; fusion; neurodegeneration; retina; retinal dystrophy; transplantation.
Copyright © 2017 The Author(s). Published by Elsevier Inc. All rights reserved.
Figures

Similar articles
-
Validating Fluorescent Chrnb4.EGFP Mouse Models for the Study of Cone Photoreceptor Degeneration.Transl Vis Sci Technol. 2020 Aug 18;9(9):28. doi: 10.1167/tvst.9.9.28. eCollection 2020 Aug. Transl Vis Sci Technol. 2020. PMID: 32879784 Free PMC article.
-
Characterization and allogeneic transplantation of a novel transgenic cone-rich donor mouse line.Exp Eye Res. 2021 Sep;210:108715. doi: 10.1016/j.exer.2021.108715. Epub 2021 Jul 31. Exp Eye Res. 2021. PMID: 34343570 Free PMC article.
-
Daylight vision repair by cell transplantation.Stem Cells. 2015 Jan;33(1):79-90. doi: 10.1002/stem.1824. Stem Cells. 2015. PMID: 25183393
-
Defective cone photoreceptor cytoskeleton, alignment, feedback, and energetics can lead to energy depletion in macular degeneration.Prog Retin Eye Res. 2004 Sep;23(5):495-522. doi: 10.1016/j.preteyeres.2004.04.005. Prog Retin Eye Res. 2004. PMID: 15302348 Review.
-
Material Exchange in Photoreceptor Transplantation: Updating Our Understanding of Donor/Host Communication and the Future of Cell Engraftment Science.Front Neural Circuits. 2018 Mar 6;12:17. doi: 10.3389/fncir.2018.00017. eCollection 2018. Front Neural Circuits. 2018. PMID: 29559897 Free PMC article. Review.
Cited by
-
Transplanted human cones incorporate into the retina and function in a murine cone degeneration model.J Clin Invest. 2022 Jun 15;132(12):e154619. doi: 10.1172/JCI154619. J Clin Invest. 2022. PMID: 35482419 Free PMC article.
-
Soluble CX3CL1 gene therapy improves cone survival and function in mouse models of retinitis pigmentosa.Proc Natl Acad Sci U S A. 2019 May 14;116(20):10140-10149. doi: 10.1073/pnas.1901787116. Epub 2019 Apr 29. Proc Natl Acad Sci U S A. 2019. PMID: 31036641 Free PMC article.
-
Rare intercellular material transfer as a confound to interpreting inner retinal neuronal transplantation following internal limiting membrane disruption.Stem Cell Reports. 2023 Nov 14;18(11):2203-2221. doi: 10.1016/j.stemcr.2023.09.005. Epub 2023 Oct 5. Stem Cell Reports. 2023. PMID: 37802075 Free PMC article.
-
Inducible Pluripotent Stem Cells to Model and Treat Inherited Degenerative Diseases of the Outer Retina: 3D-Organoids Limitations and Bioengineering Solutions.Cells. 2021 Sep 20;10(9):2489. doi: 10.3390/cells10092489. Cells. 2021. PMID: 34572137 Free PMC article. Review.
-
Development of Stem Cell Therapies for Retinal Degeneration.Cold Spring Harb Perspect Biol. 2020 Aug 3;12(8):a035683. doi: 10.1101/cshperspect.a035683. Cold Spring Harb Perspect Biol. 2020. PMID: 31818854 Free PMC article. Review.
References
-
- Roosing S., Thiadens A.A., Hoyng C.B., Klaver C.C., den Hollander A.I., Cremers F.P. Causes and consequences of inherited cone disorders. Prog. Retin. Eye Res. 2014;42:1–26. - PubMed
-
- Aït-Ali N., Fridlich R., Millet-Puel G., Clérin E., Delalande F., Jaillard C., Blond F., Perrocheau L., Reichman S., Byrne L.C. Rod-derived cone viability factor promotes cone survival by stimulating aerobic glycolysis. Cell. 2015;161:817–832. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

