Stearoyl-CoA Desaturase Promotes Liver Fibrosis and Tumor Development in Mice via a Wnt Positive-Signaling Loop by Stabilization of Low-Density Lipoprotein-Receptor-Related Proteins 5 and 6
- PMID: 28143772
- PMCID: PMC5406249
- DOI: 10.1053/j.gastro.2017.01.021
Stearoyl-CoA Desaturase Promotes Liver Fibrosis and Tumor Development in Mice via a Wnt Positive-Signaling Loop by Stabilization of Low-Density Lipoprotein-Receptor-Related Proteins 5 and 6
Abstract
Background & aims: Stearoyl-CoA desaturase (SCD) synthesizes monounsaturated fatty acids (MUFAs) and has been associated with the development of metabolic syndrome, tumorigenesis, and stem cell characteristics. We investigated whether and how SCD promotes liver fibrosis and tumor development in mice.
Methods: Rodent primary hepatic stellate cells (HSCs), mouse liver tumor-initiating stem cell-like cells (TICs), and human hepatocellular carcinoma (HCC) cell lines were exposed to Wnt signaling inhibitors and changes in gene expression patterns were analyzed. We assessed the functions of SCD by pharmacologic and conditional genetic manipulation in mice with hepatotoxic or cholestatic induction of liver fibrosis, orthotopic transplants of TICs, or liver tumors induced by administration of diethyl nitrosamine. We performed bioinformatic analyses of SCD expression in HCC vs nontumor liver samples collected from patients, and correlated levels with HCC stage and patient mortality. We performed nano-bead pull-down assays, liquid chromatography-mass spectrometry, computational modeling, and ribonucleoprotein immunoprecipitation analyses to identify MUFA-interacting proteins. We examined the effects of SCD inhibition on Wnt signaling, including the expression and stability of low-density lipoprotein-receptor-related proteins 5 and 6 (LRP5 and LRP6), by immunoblot and quantitative polymerase chain reaction analyses.
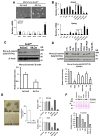
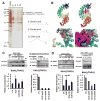
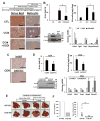
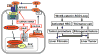
Results: SCD was overexpressed in activated HSC and HCC cells from patients; levels of SCD messenger RNA (mRNA) correlated with HCC stage and patient survival time. In rodent HSCs and TICs, the Wnt effector β-catenin increased sterol regulatory element binding protein 1-dependent transcription of Scd, and β-catenin in return was stabilized by MUFAs generated by SCD. This loop required MUFA inhibition of binding of Ras-related nuclear protein 1 (Ran1) to transportin 1 and reduced nuclear import of elav-like protein 1 (HuR), increasing cytosolic levels of HuR and HuR-mediated stabilization of mRNAs encoding LRP5 and LRP6. Genetic disruption of Scd and pharmacologic inhibitors of SCD reduced HSC activation and TIC self-renewal and attenuated liver fibrosis and tumorigenesis in mice. Conditional disruption of Scd2 in activated HSCs prevented growth of tumors from TICs and reduced the formation of diethyl nitrosamine-induced liver tumors in mice.
Conclusions: In rodent HSCs and TICs, we found SCD expression to be regulated by Wnt-β-catenin signaling, and MUFAs produced by SCD provided a forward loop to amplify Wnt signaling via stabilization of Lrp5 and Lrp6 mRNAs, contributing to liver fibrosis and tumor growth. SCD expressed by HSCs promoted liver tumor development in mice. Components of the identified loop linking HSCs and TICs might be therapeutic targets for liver fibrosis and tumors.
Keywords: Cancer Stem Cells; HCC; Hepatocarcinogenesis; HuR.
Copyright © 2017 AGA Institute. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Figures



References
-
- Fattovich G, Stroffolini T, Zagni I, et al. Hepatocellular carcinoma in cirrhosis: incidence and risk factors. Gastroenterology. 2004;127:S35–50. - PubMed
-
- Coulouarn C, Clément B. Stellate cells and the development of liver cancer: therapeutic potential of targeting the stroma. J Hepatol. 2014;60:1306–9. - PubMed
-
- Clevers H, Nusse R. Wnt/β-catenin signaling and disease. Cell. 2012;149:1192–205. - PubMed
-
- Cheng JH, She H, Han YP, et al. Wnt antagonism inhibits hepatic stellate cell activation and liver fibrosis. Am J Physiol Gastrointest Liver Physiol. 2008;294:G39–49. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

