Neutrophils acquire the capacity for antigen presentation to memory CD4+ T cells in vitro and ex vivo
- PMID: 28143882
- PMCID: PMC5383872
- DOI: 10.1182/blood-2016-10-744441
Neutrophils acquire the capacity for antigen presentation to memory CD4+ T cells in vitro and ex vivo
Abstract
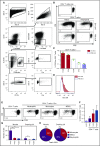
Neutrophils are critical cells of the innate immune system and rapidly respond to tissue injury and infection. Increasing evidence also indicates that neutrophils have versatile functions in contributing to adaptive immunity by internalizing and transporting antigen and influencing antigen-specific responses. Here, we demonstrate that freshly isolated human neutrophils can function as antigen-presenting cells (APCs) to memory CD4+ T cells. Neutrophils pulsed with the cognate antigens cytomegalovirus pp65 or influenza hemagglutinin were able to present the antigens to autologous antigen-specific CD4+ T cells in a major histocompatibility complex class II (MHC-II; HLA-DR)-dependent manner. Although myeloid dendritic cells and monocytes showed superior presenting ability, neutrophils consistently displayed antigen presentation capability. Upregulation of HLA-DR on neutrophils required the presence of the antigen-specific or activated T cells whereas exposure to innate stimuli such as Toll-like receptor ligands was not sufficient. Neutrophils sorted from vaccine-draining lymph nodes from rhesus macaques also showed expression of HLA-DR and were capable of presenting vaccine antigen to autologous antigen-specific memory CD4+ T cells ex vivo. Altogether, the data demonstrate that neutrophils can adapt a function as APCs and, in combination with their abundance in the immune system, may have a significant role in regulating antigen-specific T-cell responses.
Figures
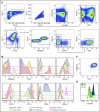

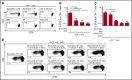
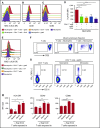
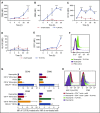
Comment in
-
Human mature neutrophils as atypical APC.Blood. 2017 Apr 6;129(14):1895-1896. doi: 10.1182/blood-2017-02-767574. Blood. 2017. PMID: 28385771 No abstract available.
References
-
- Amulic B, Cazalet C, Hayes GL, Metzler KD, Zychlinsky A. Neutrophil function: from mechanisms to disease. Annu Rev Immunol. 2012;30:459-489. - PubMed
-
- Nathan C. Neutrophils and immunity: challenges and opportunities. Nat Rev Immunol. 2006;6(3):173-182. - PubMed
-
- Thomas CJ, Schroder K. Pattern recognition receptor function in neutrophils. Trends Immunol. 2013;34(7):317-328. - PubMed
-
- Cassatella MA. Neutrophil-derived proteins: selling cytokines by the pound. Adv Immunol. 1999;73:369-509. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

