The Eph-related tyrosine kinase ligand Ephrin-B1 marks germinal center and memory precursor B cells
- PMID: 28143955
- PMCID: PMC5339677
- DOI: 10.1084/jem.20161461
The Eph-related tyrosine kinase ligand Ephrin-B1 marks germinal center and memory precursor B cells
Abstract
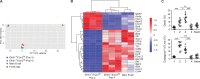
Identification of germinal center (GC) B cells is typically reliant on the use of surface activation markers that exhibit a wide range of expression. Here, we identify Ephrin-B1, a ligand for Eph-related receptor tyrosine kinases, as a specific marker of mature GC B cells. The number of Ephrin-B1+ GC B cells increases during the course of an immune response with Ephrin-B1+ GC B cells displaying elevated levels of Bcl6, S1pr2, and Aicda relative to their Ephrin-B1- counterparts. We further identified a small proportion of recently dividing, somatically mutated Ephrin-B1+ GC B cells that have begun to down-regulate Bcl6 and S1pr2 and express markers associated with memory B cells, such as CD38 and EBI2. Transcriptional analysis indicates that these cells are developmentally related to memory B cells, and likely represent a population of GC memory precursor (PreMem) B cells. GC PreMem cells display enhanced survival relative to bulk GC B cells, localize near the edge of the GC, and are predominantly found within the light zone. These findings offer insight into the significant heterogeneity that exists within the GC B cell population and provide tools to further dissect signals regulating the differentiation of GC B cells.
© 2017 Laidlaw et al.
Figures




References
-
- Bannard O., Horton R.M., Allen C.D.C., An J., Nagasawa T., and Cyster J.G.. 2013. Germinal center centroblasts transition to a centrocyte phenotype according to a timed program and depend on the dark zone for effective selection. Immunity. 39:912–924. 10.1016/j.immuni.2013.08.038 - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

