Trojan Horse Transit Contributes to Blood-Brain Barrier Crossing of a Eukaryotic Pathogen
- PMID: 28143979
- PMCID: PMC5285505
- DOI: 10.1128/mBio.02183-16
Trojan Horse Transit Contributes to Blood-Brain Barrier Crossing of a Eukaryotic Pathogen
Abstract
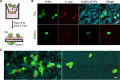
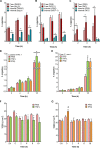
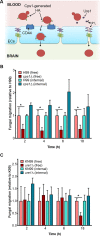
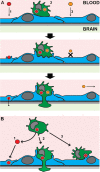
The blood-brain barrier (BBB) protects the central nervous system (CNS) by restricting the passage of molecules and microorganisms. Despite this barrier, however, the fungal pathogen Cryptococcus neoformans invades the brain, causing a meningoencephalitis that is estimated to kill over 600,000 people annually. Cryptococcal infection begins in the lung, and experimental evidence suggests that host phagocytes play a role in subsequent dissemination, although this role remains ill defined. Additionally, the disparate experimental approaches that have been used to probe various potential routes of BBB transit make it impossible to assess their relative contributions, confounding any integrated understanding of cryptococcal brain entry. Here we used an in vitro model BBB to show that a "Trojan horse" mechanism contributes significantly to fungal barrier crossing and that host factors regulate this process independently of free fungal transit. We also, for the first time, directly imaged C. neoformans-containing phagocytes crossing the BBB, showing that they do so via transendothelial pores. Finally, we found that Trojan horse crossing enables CNS entry of fungal mutants that cannot otherwise traverse the BBB, and we demonstrate additional intercellular interactions that may contribute to brain entry. Our work elucidates the mechanism of cryptococcal brain invasion and offers approaches to study other neuropathogens.
Importance: The fungal pathogen Cryptococcus neoformans invades the brain, causing a meningoencephalitis that kills hundreds of thousands of people each year. One route that has been proposed for this brain entry is a Trojan horse mechanism, whereby the fungus crosses the blood-brain barrier (BBB) as a passenger inside host phagocytes. Although indirect experimental evidence supports this intriguing mechanism, it has never been directly visualized. Here we directly image Trojan horse transit and show that it is regulated independently of free fungal entry, contributes to cryptococcal BBB crossing, and allows mutant fungi that cannot enter alone to invade the brain.
Copyright © 2017 Santiago-Tirado et al.
Figures


References
-
- Fox DS, Djordjevic JT, Sorrell TC. 2011. Signaling cascades and enzymes as cryptococcus virulence factors, p 217–234. In Heitman J, Kozel TR, Kwon-Chung KJ, Perfect J, Casadevall A (ed), Cryptococcus: from human pathogen to model yeast. ASM Press, Washington, DC.
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

