Overall survival in renal cell carcinoma after introduction of targeted therapies: a Norwegian population-based study
- PMID: 28144152
- PMCID: PMC5248939
- DOI: 10.2147/OTT.S123061
Overall survival in renal cell carcinoma after introduction of targeted therapies: a Norwegian population-based study
Abstract
Background: This population-wide retrospective, non-interventional registry study assessed changes in overall survival (OS) and factors influencing OS in Norwegian patients with renal cell carcinoma (RCC).
Methods: Two population-wide health registries were used to identify all RCC patients with (mRCC) or without metastases diagnosed before (2002-2005) and after (2006-2008 and 2009-2011) introduction of targeted therapies. Median OS was estimated using Kaplan-Meier method. Cox proportional hazards regression modeling was used to identify prognostic factors.
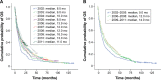
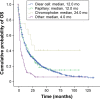
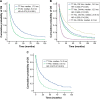
Results: Overall, 5,463 patients were diagnosed with RCC during 2002-2005 (n=1,898), 2006-2008 (n=1,631), and 2009-2011 (n=1,934); of these, 1,678 (31%) had mRCC. Patients diagnosed in 2009-2011 and 2006-2008 had significant (P<0.001) improvements in OS versus those diagnosed in 2002-2005: median OS, not reached and not reached versus 82.0 months in RCC; 14.0 and 12.0 months versus 9.0 months in mRCC. Similarly, OS improvements were seen in the primary and elderly (≥75 years) mRCC populations. Median OS was comparable (12 months) between clear cell and papillary mRCC, but it was longer (24.0 months) for chromophobe mRCC. Multivariate regression analyses showed that younger age, previous nephrectomy, and 1 or more prescriptions of targeted therapy were significantly associated with longer OS in mRCC patients.
Conclusion: OS increased in RCC and mRCC patients in Norway between 2002 and 2011 following introduction of targeted therapies.
Keywords: Norway; nephrectomy; overall survival; renal cell carcinoma; targeted therapy.
Conflict of interest statement
C Beisland has no disclosures except reimbursement of travel expenses for this study. O Klepp has received a traveling grant from Pfizer supporting presentation of the preliminary data from this study as a poster at the 2015 American Society of Clinical Oncology Genitourinary Cancers Symposium. KM Torgersen, O Solli, and R Sandin are employees of and own stock in Pfizer. J Kowalski is an independent consultant for Pfizer and is responsible for all statistical analyses for this study, for which he received funding. TB Johannesen, U Axcrona, and J Oldenburg have no conflicts of interest.
Figures

References
-
- Escudier B, Porta C, Schmidinger M, et al. Renal cell carcinoma: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2014;25(suppl 3):iii49–iii56. - PubMed
-
- Ljungberg B, Bensalah K, Canfield S, et al. EAU guidelines on renal cell carcinoma: 2014 update. Eur Urol. 2015;67(5):913–924. - PubMed
-
- National Comprehensive Cancer Network NCCN Clinical Practice Guidelines in Oncology. Kidney Cancer, Version 2.2016. [Accessed July 13, 2016]. Available from: http://www.nccn.org/professionals/physician_gls/pdf/kidney.pdf.
-
- Escudier B, Eisen T, Stadler WM, et al. Sorafenib in advanced clear-cell renal-cell carcinoma. N Engl J Med. 2007;356(2):125–134. - PubMed
-
- Motzer RJ, Hutson TE, Tomczak P, et al. Sunitinib versus interferon alfa in metastatic renal-cell carcinoma. N Engl J Med. 2007;356(2):115–124. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

