Cloning, Functional Characterization and Site-Directed Mutagenesis of 4-Coumarate: Coenzyme A Ligase (4CL) Involved in Coumarin Biosynthesis in Peucedanum praeruptorum Dunn
- PMID: 28144249
- PMCID: PMC5239791
- DOI: 10.3389/fpls.2017.00004
Cloning, Functional Characterization and Site-Directed Mutagenesis of 4-Coumarate: Coenzyme A Ligase (4CL) Involved in Coumarin Biosynthesis in Peucedanum praeruptorum Dunn
Abstract
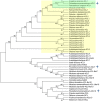
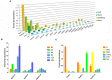
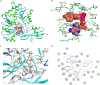
Coumarins are the main bioactive compounds in Peucedanum praeruptorum Dunn, a common Chinese herbal medicine. Nevertheless, the genes involved in the biosynthesis of core structure of coumarin in P. praeruptorum have not been identified yet. 4-Coumarate: CoA ligase (4CL) catalyzes the formation of hydroxycinnamates CoA esters, and plays an essential role at the divergence point from general phenylpropanoid metabolism to major branch pathway of coumarin. Here, three novel putative 4CL genes (Pp4CL1, Pp4CL7, and Pp4CL10) were isolated from P. praeruptorum. Biochemical characterization of the recombinant proteins revealed that Pp4CL1 utilized p-coumaric and ferulic acids as its two main substrates for coumarin biosynthesis in P. praeruptorum. Furthermore, Pp4CL1 also exhibited activity toward caffeic, cinnamic, isoferulic, and o-coumaric acids and represented a bona fide 4CL. Pp4CL7 and Pp4CL10 had no catalytic activity toward hydroxycinnamic acid compounds. But they had close phylogenetic relationship to true 4CLs and were defined as 4CL-like genes. Among all putative 4CLs, Pp4CL1 was the most highly expressed gene in roots, and its expression level was significantly up-regulated in mature roots compared with seedlings. Subcellular localization studies showed that Pp4CL1 and Pp4CL10 proteins were localized in the cytosol. In addition, site-directed mutagenesis of Pp4CL1 demonstrated that amino acids of Tyr-239, Ala-243, Met-306, Ala-309, Gly-334, Lys-441, Gln-446, and Lys-526 were essential for substrate binding or catalytic activities. The characterization and site-directed mutagenesis studies of Pp4CL1 lays a solid foundation for elucidating the biosynthetic mechanisms of coumarins in P. praeruptorum and provides further insights in understanding the structure-function relationships of this important family of proteins.
Keywords: 4-coumarate: CoA ligase; Peucedanum praeruptorum; biochemical characterization; biosynthesis mechanism; site-directed mutagenesis.
Figures





Similar articles
-
Functional characterization and correlation analysis of phenylalanine ammonia-lyase (PAL) in coumarin biosynthesis from Peucedanum praeruptorum Dunn.Phytochemistry. 2019 Feb;158:35-45. doi: 10.1016/j.phytochem.2018.11.006. Epub 2018 Nov 15. Phytochemistry. 2019. PMID: 30448740
-
Identification and functional characterization of a p-coumaroyl CoA 2'-hydroxylase involved in the biosynthesis of coumarin skeleton from Peucedanum praeruptorum Dunn.Plant Mol Biol. 2017 Sep;95(1-2):199-213. doi: 10.1007/s11103-017-0650-4. Epub 2017 Aug 18. Plant Mol Biol. 2017. PMID: 28822035
-
Two CYP71AJ enzymes function as psoralen synthase and angelicin synthase in the biosynthesis of furanocoumarins in Peucedanum praeruptorum Dunn.Plant Mol Biol. 2020 Oct;104(3):327-337. doi: 10.1007/s11103-020-01045-4. Epub 2020 Aug 6. Plant Mol Biol. 2020. PMID: 32761540
-
Structural, functional and evolutionary diversity of 4-coumarate-CoA ligase in plants.Planta. 2018 Nov;248(5):1063-1078. doi: 10.1007/s00425-018-2965-z. Epub 2018 Aug 4. Planta. 2018. PMID: 30078075 Review.
-
Trends in lignin modification: a comprehensive analysis of the effects of genetic manipulations/mutations on lignification and vascular integrity.Phytochemistry. 2002 Oct;61(3):221-94. doi: 10.1016/s0031-9422(02)00211-x. Phytochemistry. 2002. PMID: 12359514 Review.
Cited by
-
Peroxisomal Proteome Mining of Sweet Pepper (Capsicum annuum L.) Fruit Ripening Through Whole Isobaric Tags for Relative and Absolute Quantitation Analysis.Front Plant Sci. 2022 May 9;13:893376. doi: 10.3389/fpls.2022.893376. eCollection 2022. Front Plant Sci. 2022. PMID: 35615143 Free PMC article.
-
Systematic characterization of gene families and functional analysis of PvRAS3 and PvRAS4 involved in rosmarinic acid biosynthesis in Prunella vulgaris.Front Plant Sci. 2024 May 1;15:1374912. doi: 10.3389/fpls.2024.1374912. eCollection 2024. Front Plant Sci. 2024. PMID: 38751843 Free PMC article.
-
Combined metabolome and transcriptome reveal HmF6'H1 regulating simple coumarin accumulation against powdery mildew infection in Heracleum moellendorffii Hance.BMC Plant Biol. 2024 Jun 6;24(1):507. doi: 10.1186/s12870-024-05185-3. BMC Plant Biol. 2024. PMID: 38844853 Free PMC article.
-
De novo Transcriptome Sequencing Coupled With Co-expression Analysis Reveal the Transcriptional Regulation of Key Genes Involved in the Formation of Active Ingredients in Peucedanum praeruptorum Dunn Under Bolting Period.Front Genet. 2021 Jun 14;12:683037. doi: 10.3389/fgene.2021.683037. eCollection 2021. Front Genet. 2021. PMID: 34194480 Free PMC article.
-
Study of Dandelion (Taraxacum mongolicum Hand.-Mazz.) Salt Response and Caffeic Acid Metabolism under Saline Stress by Transcriptome Analysis.Genes (Basel). 2024 Feb 9;15(2):220. doi: 10.3390/genes15020220. Genes (Basel). 2024. PMID: 38397210 Free PMC article.
References
-
- Chen H., Song J., Cranos M. W., Christopher M. S., Liu J., Jack P. W., et al. (2013). Monolignol pathway 4-coumaric acid: Coenzyme A ligases in Populus trichocarpa: novel specificity, metabolic regulation, and simulation of Coenzyme A ligation fluxes. Plant Physiol. 161 1501–1516. 10.1104/pp.112.210971 - DOI - PMC - PubMed
-
- De Azevedo Souza C., Barbazuk B., Ralph S. G., Bohlmann J., Hamberger B., Douglas C. J. (2008). Genome-wide analysis of a land plant-specific acyl: Coenzyme A synthetase (ACS) gene family in Arabidopsis, poplar, rice and Physcomitrella. New Phytol. 179 987–1003. 10.1111/j.1469-8137.2008.02534.x - DOI - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

