Isosorbide and dimethyl carbonate: a green match
- PMID: 28144292
- PMCID: PMC5238621
- DOI: 10.3762/bjoc.12.218
Isosorbide and dimethyl carbonate: a green match
Abstract
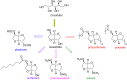
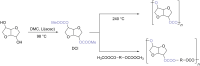
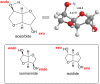
In this review the reactivity of the bio-based platform compounds D-sorbitol and isosorbide with green reagents and solvent dimethyl carbonate (DMC) is reported. Dehydration of D-sorbitol via DMC in the presence of catalytic amounts of base is an efficient and viable process for the preparation of the industrially relevant anhydro sugar isosorbide. This procedure is "chlorine-free", one-pot, environmental friendly and high yielding. The reactivity of isosorbide with DMC is equally interesting as it can lead to the formation of dicarboxymethyl isosorbide, a potential monomer for isosorbide-based polycarbonate, and dimethyl isosorbide, a high boiling green solvent. The peculiar reactivity of isosorbide and the non-toxic properties of DMC represent indeed a green match leading to several industrial appealing potential applications.
Keywords: D-sorbitol; carbohydrate chemistry; dimethyl carbonate; green chemistry; isosorbide.
Figures



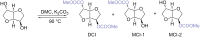


References
-
- Bozell J J, Petersen G R. Green Chem. 2010;12:539–554. doi: 10.1039/b922014c. - DOI
-
-
Despite being 15 this list is usually referred as DOE “Top 10” report.
-
-
- Werpy T, Petersen G. Results of Screening for Potential Candidates from Sugars and Synthesis Gas. I. U.S. Department of Energy; 2004. Top Value Added Chemicals from Biomass.
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
