Enduracididine, a rare amino acid component of peptide antibiotics: Natural products and synthesis
- PMID: 28144300
- PMCID: PMC5238550
- DOI: 10.3762/bjoc.12.226
Enduracididine, a rare amino acid component of peptide antibiotics: Natural products and synthesis
Abstract
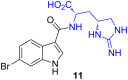
Rising resistance to current clinical antibacterial agents is an imminent threat to global public health and highlights the demand for new lead compounds for drug discovery. One such potential lead compound, the peptide antibiotic teixobactin, was recently isolated from an uncultured bacterial source, and demonstrates remarkably high potency against a wide range of resistant pathogens without apparent development of resistance. A rare amino acid residue component of teixobactin, enduracididine, is only known to occur in a small number of natural products that also possess promising antibiotic activity. This review highlights the presence of enduracididine in natural products, its biosynthesis together with a review of analogues of enduracididine. Reported synthetic approaches to the cyclic guanidine structure of enduracididine are discussed, illustrating the challenges encountered to date in the development of efficient synthetic routes to facilitate drug discovery efforts inspired by the discovery of teixobactin.
Keywords: amino acid; bacterial resistance; enduracididine; natural products; peptide antibiotics.
Figures




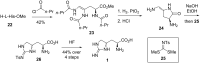











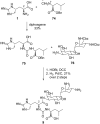
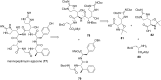
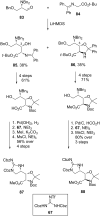









References
-
- Fellows L E, Hider R C, Bell E A. Phytochemistry. 1977;16:1957–1959. doi: 10.1016/0031-9422(77)80104-0. - DOI
-
- Evans S V, Fellows L E, Bell E A. Biochem Syst Ecol. 1985;13:271–302. doi: 10.1016/0305-1978(85)90038-9. - DOI
-
- Wilson M F, Bell E A. Phytochemistry. 1979;18:1883–1884. doi: 10.1016/0031-9422(79)83079-4. - DOI
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
