Catalytic Wittig and aza-Wittig reactions
- PMID: 28144327
- PMCID: PMC5238588
- DOI: 10.3762/bjoc.12.253
Catalytic Wittig and aza-Wittig reactions
Abstract
This review surveys the literature regarding the development of catalytic versions of the Wittig and aza-Wittig reactions. The first section summarizes how arsenic and tellurium-based catalytic Wittig-type reaction systems were developed first due to the relatively easy reduction of the oxides involved. This is followed by a presentation of the current state of the art regarding phosphine-catalyzed Wittig reactions. The second section covers the field of related catalytic aza-Wittig reactions that are catalyzed by both phosphine oxides and phosphines.
Keywords: Wittig reactions; aza-Wittig reactions; catalysis; phosphine oxides; phosphines; reduction; silanes.
Figures





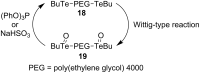
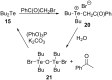


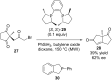




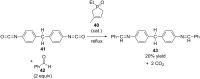

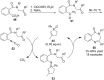
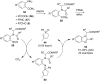



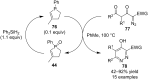
References
-
- Wittig G, Geissler G. Justus Liebigs Ann Chem. 1953;580:44–57. doi: 10.1002/jlac.19535800107. - DOI
-
- Xu S, He Z. RSC Adv. 2013;3:16885–16904. doi: 10.1039/C3RA42088D. - DOI
-
- McNulty J, McLeod D, Das P, Zepeda-Velázquez C. Phosphorus, Sulfur Silicon Relat Elem. 2015;190:619–632. doi: 10.1080/10426507.2014.980907. - DOI
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
