Development of the lung
- PMID: 28144783
- PMCID: PMC5320013
- DOI: 10.1007/s00441-016-2545-0
Development of the lung
Abstract
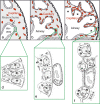
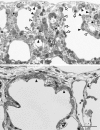
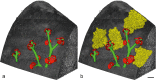
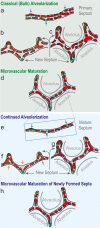
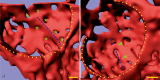
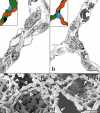
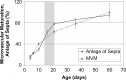
To fulfill the task of gas exchange, the lung possesses a huge inner surface and a tree-like system of conducting airways ventilating the gas exchange area. During lung development, the conducting airways are formed first, followed by the formation and enlargement of the gas exchange area. The latter (alveolarization) continues until young adulthood. During organogenesis, the left and right lungs have their own anlage, an outpouching of the foregut. Each lung bud starts a repetitive process of outgrowth and branching (branching morphogenesis) that forms all of the future airways mainly during the pseudoglandular stage. During the canalicular stage, the differentiation of the epithelia becomes visible and the bronchioalveolar duct junction is formed. The location of this junction stays constant throughout life. Towards the end of the canalicular stage, the first gas exchange may take place and survival of prematurely born babies becomes possible. Ninety percent of the gas exchange surface area will be formed by alveolarization, a process where existing airspaces are subdivided by the formation of new walls (septa). This process requires a double-layered capillary network at the basis of the newly forming septum. However, in parallel to alveolarization, the double-layered capillary network of the immature septa fuses to a single-layered network resulting in an optimized setup for gas exchange. Alveolarization still continues, because, at sites where new septa are lifting off preexisting mature septa, the required second capillary layer will be formed instantly by angiogenesis. The latter confirms a lifelong ability of alveolarization, which is important for any kind of lung regeneration.
Keywords: Alveolarization; Branching morphogenesis; Lung development; Microvascular maturation; Pulmonary acinus.
Conflict of interest statement
The author declares that he has no conflict of interest.
Figures





References
-
- Barre SF, Haberthur D, Cremona TP, Stampanoni M, Schittny JC. The total number of acini remains constant throughout postnatal rat lung development. Am J Physiol Lung Cell Mol Physiol. 2016;00325:02016. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

