Population Pharmacokinetic Model and Pharmacokinetic Target Attainment of Micafungin in Intensive Care Unit Patients
- PMID: 28144840
- PMCID: PMC5591795
- DOI: 10.1007/s40262-017-0509-5
Population Pharmacokinetic Model and Pharmacokinetic Target Attainment of Micafungin in Intensive Care Unit Patients
Abstract
Objective: To study the pharmacokinetics of micafungin in intensive care patients and assess pharmacokinetic (PK) target attainment for various dosing strategies.
Methods: Micafungin PK data from 20 intensive care unit patients were available. A population-PK model was developed. Various dosing regimens were simulated: licensed regimens (I) 100 mg daily; (II) 100 mg daily with 200 mg from day 5; and adapted regimens 200 mg on day 1 followed by (III) 100 mg daily; (IV) 150 mg daily; and (V) 200 mg daily. Target attainment based on a clinical PK target for Candida as well as non-Candida parapsilosis infections was assessed for relevant minimum inhibitory concentrations [MICs] (Clinical and Laboratory Standards Institute). Parameter uncertainty was taken into account in simulations.
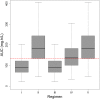
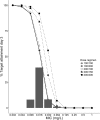
Results: A two-compartment model best fitted the data. Clearance was 1.10 (root square error 8%) L/h and V 1 and V 2 were 17.6 (root square error 14%) and 3.63 (root square error 8%) L, respectively. Median area under the concentration-time curve over 24 h (interquartile range) on day 14 for regimens I-V were 91 (67-122), 183 (135-244), 91 (67-122), 137 (101-183) and 183 (135-244) mg h/L, respectively, for a typical patient of 70 kg. For the MIC/area under the concentration-time curve >3000 target (all Candida spp.), PK target attainment was >91% on day 14 (MIC 0.016 mg/L epidemiological cut-off) for all of the dosing regimens but decreased to (I) 44%, (II) 91%, (III) 44%, (IV) 78% and (V) 91% for MIC 0.032 mg/L. For the MIC/area under the concentration-time curve >5000 target (non-C. parapsilosis spp.), PK target attainment varied between 62 and 96% on day 14 for MIC 0.016.
Conclusions: The licensed micafungin maintenance dose results in adequate exposure based on our simulations with a clinical PK target for Candida infections but only 62% of patients reach the target for non-C. parapsilosis. In the case of pathogens with an attenuated micafungin MIC, patients may benefit from dose escalation to 200 mg daily. This encourages future study.
Keywords: Intensive Care Unit Patient; Invasive Candidiasis; Micafungin; Sequential Organ Failure Assessment; Sequential Organ Failure Assessment Score.
Conflict of interest statement
Funding
The original study published by Lempers et al. was supported by an unrestricted educational grant from Astellas.
Conflict of interest
R. J. Brüggemann has served as a consultant to and has received unrestricted and research grants from Astellas Pharma, Inc., Gilead Sciences, Merck Sharpe and Dohme Corp., and Pfizer, Inc. P. E. Verweij has served as a consultant to and has received unrestricted and research grants from Astellas Pharma, Inc., Gilead Sciences, Merck Sharpe and Dohme Corp., and Pfizer, Inc., F2G Ltd. J. A. Schouten has received support for educational activities from Astellas and Merck Sharpe and Dohme.
Figures

References
-
- European Medicines Agency. Mycamine: EPAR scientific discussion. 2008. http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Public_asses.... Accessed 2017 Jan 19.
-
- European Medicines Agency. Summary of product characteristics: mycamine. 2009. http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Info.... Accessed 2017 Jan 19.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

