Transplanted miR-219-overexpressing oligodendrocyte precursor cells promoted remyelination and improved functional recovery in a chronic demyelinated model
- PMID: 28145507
- PMCID: PMC5286453
- DOI: 10.1038/srep41407
Transplanted miR-219-overexpressing oligodendrocyte precursor cells promoted remyelination and improved functional recovery in a chronic demyelinated model
Erratum in
-
Author Correction: Transplanted miR-219-overexpressing oligodendrocyte precursor cells promoted remyelination and improved functional recovery in a chronic demyelinated model.Sci Rep. 2018 Oct 29;8(1):15848. doi: 10.1038/s41598-018-34063-w. Sci Rep. 2018. PMID: 30374121 Free PMC article.
Abstract
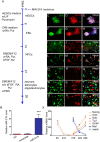
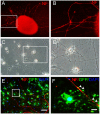
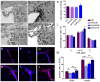
Oligodendrocyte precursor cells (OPCs) have the ability to repair demyelinated lesions by maturing into myelin-producing oligodendrocytes. Recent evidence suggests that miR-219 helps regulate the differentiation of OPCs into oligodendrocytes. We performed oligodendrocyte differentiation studies using miR-219-overexpressing mouse embryonic stem cells (miR219-mESCs). The self-renewal and multiple differentiation properties of miR219-mESCs were analyzed by the expression of the stage-specific cell markers Nanog, Oct4, nestin, musashi1, GFAP, Tuj1 and O4. MiR-219 accelerated the differentiation of mESC-derived neural precursor cells (NPCs) into OPCs. We further transplanted OPCs derived from miR219-mESCs (miR219-OPCs) into cuprizone-induced chronically demyelinated mice to observe remyelination, which resulted in well-contained oligodendrocyte grafts that migrated along the corpus callosum and matured to express myelin basic protein (MBP). Ultrastructural studies further confirmed the presence of new myelin sheaths. Improved cognitive function in these mice was confirmed by behavioral tests. Importantly, the transplanted miR219-OPCs induced the proliferation of endogenous NPCs. In conclusion, these data demonstrate that miR-219 rapidly transforms mESCs into oligodendrocyte lineage cells and that the transplantation of miR219-OPCs not only promotes remyelination and improves cognitive function but also enhances the proliferation of host endogenous NPCs following chronic demyelination. These results support the potential of a therapeutic role for miR-219 in demyelinating diseases.
Conflict of interest statement
The authors declare no competing financial interests.
Figures




Similar articles
-
Inactivation of Protein Tyrosine Phosphatase Receptor Type Z by Pleiotrophin Promotes Remyelination through Activation of Differentiation of Oligodendrocyte Precursor Cells.J Neurosci. 2015 Sep 2;35(35):12162-71. doi: 10.1523/JNEUROSCI.2127-15.2015. J Neurosci. 2015. PMID: 26338327 Free PMC article.
-
Experimental Demyelination and Axonal Loss Are Reduced in MicroRNA-146a Deficient Mice.Front Immunol. 2018 Mar 12;9:490. doi: 10.3389/fimmu.2018.00490. eCollection 2018. Front Immunol. 2018. PMID: 29593734 Free PMC article.
-
miR-219 attenuates demyelination in cuprizone-induced demyelinated mice by regulating monocarboxylate transporter 1.Eur J Neurosci. 2017 Jan;45(2):249-259. doi: 10.1111/ejn.13485. Eur J Neurosci. 2017. PMID: 27873367
-
Engineering biomaterial microenvironments to promote myelination in the central nervous system.Brain Res Bull. 2019 Oct;152:159-174. doi: 10.1016/j.brainresbull.2019.07.013. Epub 2019 Jul 12. Brain Res Bull. 2019. PMID: 31306690 Review.
-
Oligodendrogenesis from neural stem cells: perspectives for remyelinating strategies.Int J Dev Neurosci. 2013 Nov;31(7):692-700. doi: 10.1016/j.ijdevneu.2013.01.004. Epub 2013 Jan 20. Int J Dev Neurosci. 2013. PMID: 23340483 Review.
Cited by
-
MS CD49d+CD154+ Lymphocytes Reprogram Oligodendrocytes into Immune Reactive Cells Affecting CNS Regeneration.Cells. 2019 Nov 25;8(12):1508. doi: 10.3390/cells8121508. Cells. 2019. PMID: 31775315 Free PMC article.
-
Relationship between sex biases in gene expression and sex biases in autism and Alzheimer's disease.medRxiv [Preprint]. 2023 Sep 1:2023.08.29.23294773. doi: 10.1101/2023.08.29.23294773. medRxiv. 2023. Update in: Biol Sex Differ. 2024 Jun 7;15(1):47. doi: 10.1186/s13293-024-00622-2. PMID: 37693465 Free PMC article. Updated. Preprint.
-
Identification of genome-wide targets of Olig2 in the adult mouse spinal cord using ChIP-Seq.PLoS One. 2017 Oct 19;12(10):e0186091. doi: 10.1371/journal.pone.0186091. eCollection 2017. PLoS One. 2017. PMID: 29049317 Free PMC article.
-
Fully Characterized Mature Human iPS- and NMP-Derived Motor Neurons Thrive Without Neuroprotection in the Spinal Contusion Cavity.Front Cell Neurosci. 2022 Jan 3;15:725195. doi: 10.3389/fncel.2021.725195. eCollection 2021. Front Cell Neurosci. 2022. PMID: 35046774 Free PMC article.
-
[Musashi-1 positively regulates growth and proliferation of hepatoma cells in vitro].Nan Fang Yi Ke Da Xue Xue Bao. 2019 Dec 30;39(12):1436-1442. doi: 10.12122/j.issn.1673-4254.2019.12.07. Nan Fang Yi Ke Da Xue Xue Bao. 2019. PMID: 31907147 Free PMC article. Chinese.
References
-
- Chang A., Tourtellotte W. W., Rudick R. & Trapp B. D. Premyelinating oligodendrocytes in chronic lesions of multiple sclerosis. N. Engl.J. Med. 346, 165–173 (2002). - PubMed
-
- Foote A. K. & Blakemore W. F. Inflammation stimulates remyelination in areas of chronic demyelination. Brain 128, 528–39 (2005). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

