Role of Quinone Reductase 2 in the Antimalarial Properties of Indolone-Type Derivatives
- PMID: 28146103
- PMCID: PMC6155775
- DOI: 10.3390/molecules22020210
Role of Quinone Reductase 2 in the Antimalarial Properties of Indolone-Type Derivatives
Abstract
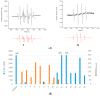
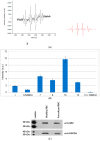
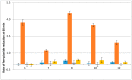
Indolone-N-oxides have antiplasmodial properties against Plasmodium falciparum at the erythrocytic stage, with IC50 values in the nanomolar range. The mechanism of action of indolone derivatives involves the production of free radicals, which follows their bioreduction by an unknown mechanism. In this study, we hypothesized that human quinone reductase 2 (hQR2), known to act as a flavin redox switch upon binding to the broadly used antimalarial chloroquine, could be involved in the activity of the redox-active indolone derivatives. Therefore, we investigated the role of hQR2 in the reduction of indolone derivatives. We analyzed the interaction between hQR2 and several indolone-type derivatives by examining enzymatic kinetics, the substrate/protein complex structure with X-ray diffraction analysis, and the production of free radicals with electron paramagnetic resonance. The reduction of each compound in cells overexpressing hQR2 was compared to its reduction in naïve cells. This process could be inhibited by the specific hQR2 inhibitor, S29434. These results confirmed that the anti-malarial activity of indolone-type derivatives was linked to their ability to serve as hQR2 substrates and not as hQR2 inhibitors as reported for chloroquine, leading to the possibility that substrate of hQR2 could be considered as a new avenue for the design of new antimalarial compounds.
Keywords: human quinone reductase 2; indolones; inhibitor; malaria; mechanism.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
Antimalarial Properties of Dunnione Derivatives as NQO2 Substrates.Molecules. 2019 Oct 15;24(20):3697. doi: 10.3390/molecules24203697. Molecules. 2019. PMID: 31618826 Free PMC article.
-
Indolone-N-oxide derivatives: in vitro activity against fresh clinical isolates of Plasmodium falciparum, stage specificity and in vitro interactions with established antimalarial drugs.J Antimicrob Chemother. 2011 Nov;66(11):2566-72. doi: 10.1093/jac/dkr320. Epub 2011 Aug 22. J Antimicrob Chemother. 2011. PMID: 21862474
-
Amino derivatives of indolone-N-oxide: preparation and antiplasmodial properties.Eur J Med Chem. 2014 Apr 9;76:369-75. doi: 10.1016/j.ejmech.2014.02.038. Epub 2014 Feb 14. Eur J Med Chem. 2014. PMID: 24594524
-
Antimalarial activities of indolones and derivatives.Curr Top Med Chem. 2014;14(14):1643-52. doi: 10.2174/1568026614666140808121329. Curr Top Med Chem. 2014. PMID: 25116584 Review.
-
Antimalarial drugs inhibiting hemozoin (beta-hematin) formation: a mechanistic update.Life Sci. 2007 Feb 6;80(9):813-28. doi: 10.1016/j.lfs.2006.11.008. Epub 2006 Nov 10. Life Sci. 2007. PMID: 17157328 Review.
Cited by
-
An LC-MS Assay to Measure Superoxide Radicals and Hydrogen Peroxide in the Blood System.Metabolites. 2020 Apr 28;10(5):175. doi: 10.3390/metabo10050175. Metabolites. 2020. PMID: 32354089 Free PMC article.
-
Antimalarial Properties of Dunnione Derivatives as NQO2 Substrates.Molecules. 2019 Oct 15;24(20):3697. doi: 10.3390/molecules24203697. Molecules. 2019. PMID: 31618826 Free PMC article.
References
-
- WHO World Malaria Report. [(accessed on 14 April 2016)]. Available online: http://www.who.int/malaria/publications/world-malaria-report-2015/report/en.
-
- Medicines for Malaria Venture (MMV) [(accessed on 14 April 2016)]. Available online: http://www.mmv.org/research-development/interactive-rd-portfolio.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

