Polyphenolic Extract of Euphorbia supina Attenuates Manganese-Induced Neurotoxicity by Enhancing Antioxidant Activity through Regulation of ER Stress and ER Stress-Mediated Apoptosis
- PMID: 28146110
- PMCID: PMC5343836
- DOI: 10.3390/ijms18020300
Polyphenolic Extract of Euphorbia supina Attenuates Manganese-Induced Neurotoxicity by Enhancing Antioxidant Activity through Regulation of ER Stress and ER Stress-Mediated Apoptosis
Abstract
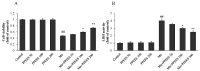
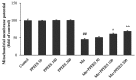
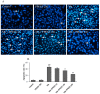
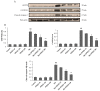
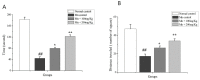
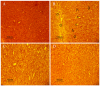
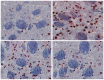
Manganese (Mn) is an important trace element present in human body, which acts as an enzyme co-factor or activator in various metabolic reactions. While essential in trace amounts, excess levels of Mn in human brain can produce neurotoxicity, including idiopathic Parkinson's disease (PD)-like extrapyramidal manganism symptoms. This study aimed to investigate the protective role of polyphenolic extract of Euphorbia supina (PPEES) on Mn-induced neurotoxicity and the underlying mechanism in human neuroblastoma SKNMC cells and Sprague-Dawley (SD) male rat brain. PPEES possessed significant amount of total phenolic and flavonoid contents. PPEES also showed significant antioxidant activity in 1,1-diphenyl-2-picrylhydrazyl (DPPH) radical scavenging and reducing power capacity (RPC) assays. Our results showed that Mn treatment significantly reduced cell viability and increased lactate dehydrogenase (LDH) level, which was attenuated by PPEES pretreatment at 100 and 200 µg/mL. Additionally, PPEES pretreatment markedly attenuated Mn-induced antioxidant status alteration by resolving the ROS, MDA and GSH levels and SOD and CAT activities. PPEES pretreatment also significantly attenuated Mn-induced mitochondrial membrane potential (ΔΨm) and apoptosis. Meanwhile, PPEES pretreatment significantly reversed the Mn-induced alteration in the GRP78, GADD34, XBP-1, CHOP, Bcl-2, Bax and caspase-3 activities. Furthermore, administration of PPEES (100 and 200 mg/kg) to Mn exposed rats showed improvement of histopathological alteration in comparison to Mn-treated rats. Moreover, administration of PPEES to Mn exposed rats showed significant reduction of 8-OHdG and Bax immunoreactivity. The results suggest that PPEES treatment reduces Mn-induced oxidative stress and neuronal cell loss in SKNMC cells and in the rat brain. Therefore, PPEES may be considered as potential treat-ment in Mn-intoxicated patients.
Keywords: Euphorbia supina; antioxidant; manganese; neuroprotection; neurotoxicity.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
-
- Stephenson A.P., Schneider J.A., Nelson B.C., Atha D.H., Jain A., Soliman K.F., Aschner M., Mazzio E., Renee Reams R. Manganese-induced oxidative DNA damage in neuronal SH-SY5Y cells: Attenuation of thymine base lesions by glutathione and N-acetylcysteine. Toxicol. Lett. 2013;218:299–307. doi: 10.1016/j.toxlet.2012.12.024. - DOI - PMC - PubMed
-
- Hudnell H.K. Effects from environmental Mn exposures: A review of the evidence from non-occupational exposure studies. Neurotoxicology. 1999;20:379–397. - PubMed
-
- Barbeau A. Manganese and extrapyramidal disorders (a critical review and tribute to Dr. George C. Cotzias) Neurotoxicology. 1984;5:13–35. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

