A Novel Convergent Synthesis of the Potent Antiglaucoma Agent Tafluprost
- PMID: 28146132
- PMCID: PMC6155834
- DOI: 10.3390/molecules22020217
A Novel Convergent Synthesis of the Potent Antiglaucoma Agent Tafluprost
Abstract
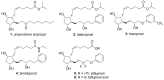
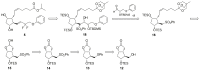
Tafluprost (AFP-168, 5) is a unique 15-deoxy-15,15-difluoro-16-phenoxy prostaglandin F2α (PGF2α) analog used as an efficacious ocular hypotensive agent in the treatment of glaucoma and ocular hypertension, as monotherapy, or as adjunctive therapy to β-blockers. A novel convergent synthesis of 5 was developed employing Julia-Lythgoe olefination of the structurally advanced prostaglandin phenylsulfone 16, also successfully applied for manufacturing of pharmaceutical grade latanoprost (2), travoprost (3) and bimatoprost (4), with an aldehyde ω-chain synthon 17. The use of the same prostaglandin phenylsulfone 16, as a starting material in parallel syntheses of all commercially available antiglaucoma PGF2α analogs 2-5, significantly reduces manufacturing costs resulting from its synthesis on an industrial scale and development of technological documentation. Another key aspect of the route developed is deoxydifluorination of a trans-13,14-en-15-one 30 with Deoxo-Fluor. Subsequent hydrolysis of protecting groups and final esterification of acid 6 yielded tafluprost (5). The main advantages are the preparation of high purity tafluprost (5) and the application of comparatively cheap reagents. The preparation and identification of two other tafluprost acid derivatives, tafluprost methyl ester (32) and tafluprost ethyl amide (33), are also described.
Keywords: Corey lactone; Julia–Lythgoe olefination; fluorination; prostaglandins; tafluprost.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



Similar articles
-
A novel convergent synthesis of the antiglaucoma PGF2α analogue bimatoprost.Chirality. 2013 Mar;25(3):170-9. doi: 10.1002/chir.22123. Epub 2013 Feb 5. Chirality. 2013. PMID: 23381781
-
Therapeutic uses of prostaglandin F(2α) analogues in ocular disease and novel synthetic strategies.Prostaglandins Other Lipid Mediat. 2013 Jul-Aug;104-105:109-21. doi: 10.1016/j.prostaglandins.2013.01.001. Epub 2013 Jan 23. Prostaglandins Other Lipid Mediat. 2013. PMID: 23353557
-
Prostaglandin analogues and mouse intraocular pressure: effects of tafluprost, latanoprost, travoprost, and unoprostone, considering 24-hour variation.Invest Ophthalmol Vis Sci. 2005 Jun;46(6):2006-11. doi: 10.1167/iovs.04-1527. Invest Ophthalmol Vis Sci. 2005. PMID: 15914616
-
Tafluprost: the first preservative-free prostaglandin to treat open-angle glaucoma and ocular hypertension.Ann Pharmacother. 2012 Nov;46(11):1506-10. doi: 10.1345/aph.1R229. Epub 2012 Oct 23. Ann Pharmacother. 2012. PMID: 23092867 Review.
-
Prostaglandin analog treatment of glaucoma and ocular hypertension.Ann Pharmacother. 2002 Mar;36(3):504-11. doi: 10.1345/aph.1A178. Ann Pharmacother. 2002. PMID: 11895065 Review.
Cited by
-
A concise and scalable chemoenzymatic synthesis of prostaglandins.Nat Commun. 2024 Mar 21;15(1):2523. doi: 10.1038/s41467-024-46960-y. Nat Commun. 2024. PMID: 38514642 Free PMC article.
-
3,3-Difluoroallyl ammonium salts: highly versatile, stable and selective gem-difluoroallylation reagents.Nat Commun. 2021 May 31;12(1):3257. doi: 10.1038/s41467-021-23504-2. Nat Commun. 2021. PMID: 34059673 Free PMC article.
References
-
- Piplani P., Aggarwal D., Abbhi V., Saini L. Prostaglandin analogues: Current treatment opinion for glaucoma. Med. Chem. Res. 2016;25:1031–1048. doi: 10.1007/s00044-016-1563-5. - DOI
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

