The HDAC inhibitor AR42 interacts with pazopanib to kill trametinib/dabrafenib-resistant melanoma cells in vitro and in vivo
- PMID: 28146421
- PMCID: PMC5369969
- DOI: 10.18632/oncotarget.14829
The HDAC inhibitor AR42 interacts with pazopanib to kill trametinib/dabrafenib-resistant melanoma cells in vitro and in vivo
Abstract
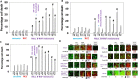
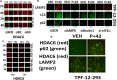
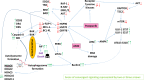
Studies focused on the killing of activated B-RAF melanoma cells by the histone deacetylase (HDAC) inhibitor AR42. Compared to other tumor cell lines, PDX melanoma isolates were significantly more sensitive to AR42-induced killing. AR42 and the multi-kinase inhibitor pazopanib interacted to activate: an eIF2α-Beclin1 pathway causing autophagosome formation; an eIF2α-DR4/DR5/CD95 pathway; and an eIF2α-dependent reduction in the expression of c-FLIP-s, MCL-1 and BCL-XL. AR42 did not alter basal chaperone activity but increased the ability of pazopanib to inhibit HSP90, HSP70 and GRP78. AR42 and pazopanib caused HSP90/HSP70 dissociation from RAF-1 and B-RAF that resulted in reduced 'RAF' expression. The drug combination activated a DNA-damage-ATM-AMPK pathway that was associated with: NFκB activation; reduced mTOR S2448 and ULK-1 S757 phosphorylation; and increased ULK-1 S317 and ATG13 S318 phosphorylation. Knock down of PERK, eIF2α, Beclin1, ATG5 or AMPKα, or expression of IκB S32A S36A, ca-mTOR or TRX, reduced cell killing. AR42, via lysosomal degradation, reduced the protein expression of HDACs 2/5/6/10/11. In vivo, a 3-day exposure of dabrafenib/trametinib resistant melanoma cells to the AR42 pazopanib combination reduced tumor growth and enhanced survival from ~25 to ~40 days. Tumor cells that had adapted through therapy exhibited elevated HGF expression and the c-MET inhibitor crizotinib enhanced AR42 pazopanib lethality in this evolved drug-resistant population.
Keywords: ER stress; autophagy; chaperone; death receptor.
Conflict of interest statement
The authors have no conflicts of interest to report.
Figures











References
-
- Park MA, Mitchell C, Zhang G, Yacoub A, Allegood J, Häussinger D, Reinehr R, Larner A, Spiegel S, Fisher PB, Voelkel-Johnson C, Ogretmen B, Grant S, et al. Vorinostat and sorafenib increase CD95 activation in gastrointestinal tumor cells through a Ca(2+)-de novo ceramide-PP2A-reactive oxygen species-dependent signaling pathway. Cancer Res. 2010;70:6313–24. - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

