Enhanced Fine-Form Perception Does Not Contribute to Gestalt Face Perception in Autism Spectrum Disorder
- PMID: 28146575
- PMCID: PMC5287487
- DOI: 10.1371/journal.pone.0170239
Enhanced Fine-Form Perception Does Not Contribute to Gestalt Face Perception in Autism Spectrum Disorder
Abstract
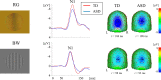
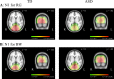
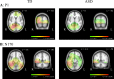
Individuals with autism spectrum disorder (ASD) show superior performance in processing fine detail, but often exhibit impaired gestalt face perception. The ventral visual stream from the primary visual cortex (V1) to the fusiform gyrus (V4) plays an important role in form (including faces) and color perception. The aim of this study was to investigate how the ventral stream is functionally altered in ASD. Visual evoked potentials were recorded in high-functioning ASD adults (n = 14) and typically developing (TD) adults (n = 14). We used three types of visual stimuli as follows: isoluminant chromatic (red/green, RG) gratings, high-contrast achromatic (black/white, BW) gratings with high spatial frequency (HSF, 5.3 cycles/degree), and face (neutral, happy, and angry faces) stimuli. Compared with TD controls, ASD adults exhibited longer N1 latency for RG, shorter N1 latency for BW, and shorter P1 latency, but prolonged N170 latency, for face stimuli. Moreover, a greater difference in latency between P1 and N170, or between N1 for BW and N170 (i.e., the prolongation of cortico-cortical conduction time between V1 and V4) was observed in ASD adults. These findings indicate that ASD adults have enhanced fine-form (local HSF) processing, but impaired color processing at V1. In addition, they exhibit impaired gestalt face processing due to deficits in integration of multiple local HSF facial information at V4. Thus, altered ventral stream function may contribute to abnormal social processing in ASD.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures



Similar articles
-
Connectopathy in Autism Spectrum Disorders: A Review of Evidence from Visual Evoked Potentials and Diffusion Magnetic Resonance Imaging.Front Neurosci. 2017 Nov 9;11:627. doi: 10.3389/fnins.2017.00627. eCollection 2017. Front Neurosci. 2017. PMID: 29170625 Free PMC article. Review.
-
Slow segmentation of faces in Autism Spectrum Disorder.Neuropsychologia. 2019 Apr;127:1-8. doi: 10.1016/j.neuropsychologia.2019.02.005. Epub 2019 Feb 12. Neuropsychologia. 2019. PMID: 30768937
-
Enhanced Early Visual Responses During Implicit Emotional Faces Processing in Autism Spectrum Disorder.J Autism Dev Disord. 2019 Mar;49(3):871-886. doi: 10.1007/s10803-018-3787-3. J Autism Dev Disord. 2019. PMID: 30374763
-
Testing the effects of expression, intensity and age on emotional face processing in ASD.Neuropsychologia. 2019 Mar 18;126:128-137. doi: 10.1016/j.neuropsychologia.2017.06.023. Epub 2017 Jun 21. Neuropsychologia. 2019. PMID: 28647439
-
Distributed and interactive brain mechanisms during emotion face perception: evidence from functional neuroimaging.Neuropsychologia. 2007 Jan 7;45(1):174-94. doi: 10.1016/j.neuropsychologia.2006.06.003. Epub 2006 Jul 18. Neuropsychologia. 2007. PMID: 16854439 Review.
Cited by
-
Connectopathy in Autism Spectrum Disorders: A Review of Evidence from Visual Evoked Potentials and Diffusion Magnetic Resonance Imaging.Front Neurosci. 2017 Nov 9;11:627. doi: 10.3389/fnins.2017.00627. eCollection 2017. Front Neurosci. 2017. PMID: 29170625 Free PMC article. Review.
-
Atypical visual processing in a mouse model of autism.Sci Rep. 2020 Jul 24;10(1):12390. doi: 10.1038/s41598-020-68589-9. Sci Rep. 2020. PMID: 32709898 Free PMC article.
-
Electrophysiological Studies of Reception of Facial Communication in Autism Spectrum Disorder and Schizophrenia.Rev J Autism Dev Disord. 2022 Dec;9(4):521-554. doi: 10.1007/s40489-021-00260-z. Epub 2021 Jun 25. Rev J Autism Dev Disord. 2022. PMID: 36568688 Free PMC article.
References
-
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders 5th ed. Washington, DC: APA; 2013.
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

