Prolonged use of the etonogestrel implant and levonorgestrel intrauterine device: 2 years beyond Food and Drug Administration-approved duration
- PMID: 28147241
- PMCID: PMC5449254
- DOI: 10.1016/j.ajog.2017.01.036
Prolonged use of the etonogestrel implant and levonorgestrel intrauterine device: 2 years beyond Food and Drug Administration-approved duration
Abstract
Background: The subdermal contraceptive implant and the 52-mg levonorgestrel intrauterine device are currently Food and Drug Administration approved for 3 and 5 years of use, respectively. Limited available data suggested both of these methods are effective beyond that time. Demonstration of prolonged effectiveness will improve the cost-effectiveness of the device, and potentially patient continuation and satisfaction.
Objective: We sought to evaluate the effectiveness of the contraceptive implant and the 52-mg hormonal intrauterine device in women using the method for 2 years beyond the current Food and Drug Administration-approved duration.
Study design: We initiated this ongoing prospective cohort study in January 2012. We are enrolling women using the contraceptive implant or 52-mg levonorgestrel intrauterine device for a minimum of 3 and 5 years, respectively (started intrauterine device in ≥2007 or implant in ≥2009). Demographic and reproductive health histories, as well as objective body mass index, were collected. Implant users were offered periodic venipuncture for analysis of serum etonogestrel levels. The primary outcome, unintended pregnancy rate, was calculated per 100 woman-years. We analyzed baseline demographic characteristics using χ2 test and Fisher exact test, and compared serum etonogestrel levels stratified by body mass index using the Kruskal-Wallis test.
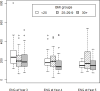
Results: Implant users (n = 291) have contributed 444.0 woman-years of follow-up. There have been no documented pregnancies in implant users during the 2 years of postexpiration follow-up. Calculated failure rates in the fourth and fifth years for the implant are calculated as 0 (1-sided 97.5% confidence interval, 0-1.48) per 100 woman-years at 4 years and 0 (1-sided 97.5% confidence interval, 0-2.65) per 100 woman-years at 5 years. Among 496 levonorgestrel intrauterine device users, 696.9 woman-years of follow-up have been completed. Two pregnancies have been reported. The failure rate in the sixth year of use of the levonorgestrel intrauterine device is calculated as 0.25 (95% confidence interval, 0.04-1.42) per 100 woman-years; failure rate during the seventh year is 0.43 (95% confidence interval, 0.08-2.39) per 100 woman-years. Among implant users with serum etonogestrel results, the median etonogestrel level was 207.7 pg/mL (range 63.8-802.6 pg/mL) at the time of method expiration, 166.1 pg/mL (range 67.9 25.0-470.5 pg/mL) at the end of the fourth year, and 153.0 pg/mL (range 72.1-538.8 pg/mL) at the end of the fifth year. Median etonogestrel levels were compared by body mass index at each time point and a statistical difference was noted at the end of 4 years of use with overweight women having the highest serum etonogestrel (195.9; range 25.0-450.5 pg/mL) when compared to normal (178.9; range 87.0-463.7 pg/mL) and obese (137.9; range 66.0-470.5 pg/mL) women (P = .04).
Conclusion: This study indicates that the contraceptive implant and 52-mg hormonal intrauterine device continue to be highly effective for at least 2 additional years of use. Serum etonogestrel evaluation demonstrates median levels remain above the ovulation threshold of 90 pg/mL for women in all body mass index classes.
Keywords: Food and Drug Administration–approved duration; effectiveness; implant; intrauterine device; prolonged use.
Copyright © 2017 Elsevier Inc. All rights reserved.
Figures
Similar articles
-
Use of the etonogestrel implant and levonorgestrel intrauterine device beyond the U.S. Food and Drug Administration-approved duration.Obstet Gynecol. 2015 Mar;125(3):599-604. doi: 10.1097/AOG.0000000000000690. Obstet Gynecol. 2015. PMID: 25730221 Free PMC article.
-
A 3-year multicentre randomized controlled trial of etonogestrel- and levonorgestrel-releasing contraceptive implants, with non-randomized matched copper-intrauterine device controls.Hum Reprod. 2015 Nov;30(11):2527-38. doi: 10.1093/humrep/dev221. Epub 2015 Sep 25. Hum Reprod. 2015. PMID: 26409014 Clinical Trial.
-
Extended use up to 5 years of the etonogestrel-releasing subdermal contraceptive implant: comparison to levonorgestrel-releasing subdermal implant.Hum Reprod. 2016 Nov;31(11):2491-2498. doi: 10.1093/humrep/dew222. Epub 2016 Sep 26. Hum Reprod. 2016. PMID: 27671673 Free PMC article. Clinical Trial.
-
Subdermal contraceptive implants.J Steroid Biochem Mol Biol. 1995 Jun;53(1-6):223-6. doi: 10.1016/0960-0760(95)00051-z. J Steroid Biochem Mol Biol. 1995. PMID: 7626459 Review.
-
Risks and benefits, advantages and disadvantages of levonorgestrel-releasing contraceptive implants.Drug Saf. 2003;26(5):303-35. doi: 10.2165/00002018-200326050-00002. Drug Saf. 2003. PMID: 12650633 Review.
Cited by
-
Providing Contraception for Young People During a Pandemic Is Essential Health Care.JAMA Pediatr. 2020 Sep 1;174(9):823-824. doi: 10.1001/jamapediatrics.2020.1884. JAMA Pediatr. 2020. PMID: 32379298 Free PMC article. Review. No abstract available.
-
Expanding choice and access in contraception: an assessment of intrauterine contraception policies in low and middle-income countries.BMC Public Health. 2019 Dec 19;19(1):1707. doi: 10.1186/s12889-019-8080-7. BMC Public Health. 2019. PMID: 31856766 Free PMC article.
-
Consecutive Use of the 52 mg Levonorgestrel-releasing Intrauterine System: Variations in Bleeding Patterns.Rev Bras Ginecol Obstet. 2020 Apr;42(4):194-199. doi: 10.1055/s-0040-1708092. Epub 2020 Apr 24. Rev Bras Ginecol Obstet. 2020. PMID: 32330961 Free PMC article.
-
Offering extended use of the contraceptive implant via an implementation science framework: a qualitative study of clinicians' perceived barriers and facilitators.BMC Health Serv Res. 2024 Jun 3;24(1):697. doi: 10.1186/s12913-024-10991-4. BMC Health Serv Res. 2024. PMID: 38825705 Free PMC article.
-
Etonogestrel implant failure in a woman taking thyroid hormone replacement: A case report.Case Rep Womens Health. 2025 Jan 17;45:e00687. doi: 10.1016/j.crwh.2025.e00687. eCollection 2025 Mar. Case Rep Womens Health. 2025. PMID: 39901894 Free PMC article.
References
-
- Daniels K, Daugherty J, Jones J. Current contraceptive status among women aged 15–44: United States, 2011–2013. NCHS data brief. 2014;(173):1–8. - PubMed
-
- Sivin I. Long-term use of copper intrauterine devices. Lancet. 1990;336(8713):504. - PubMed
-
- Kiriwat O, Patanayindee A, Koetsawang S, Korver T, Bennink HJ. A 4-year pilot study on the efficacy and safety of Implanon, a single-rod hormonal contraceptive implant, in healthy women in Thailand. Eur J Contracept Reprod Health CAre. 1998 Jun;3(2):85–91. - PubMed
-
- Wu JP, Pickle S. Extended use of the intrauterine device: a literature review and recommendations for clinical practice. Contraception. 2014;89(6):495–503. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources