Neuregulin 3 Mediates Cortical Plate Invasion and Laminar Allocation of GABAergic Interneurons
- PMID: 28147272
- PMCID: PMC5300889
- DOI: 10.1016/j.celrep.2016.12.089
Neuregulin 3 Mediates Cortical Plate Invasion and Laminar Allocation of GABAergic Interneurons
Abstract
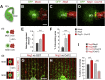
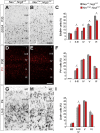
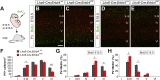
Neural circuits in the cerebral cortex consist of excitatory pyramidal cells and inhibitory interneurons. These two main classes of cortical neurons follow largely different genetic programs, yet they assemble into highly specialized circuits during development following a very precise choreography. Previous studies have shown that signals produced by pyramidal cells influence the migration of cortical interneurons, but the molecular nature of these factors has remained elusive. Here, we identified Neuregulin 3 (Nrg3) as a chemoattractive factor expressed by developing pyramidal cells that guides the allocation of cortical interneurons in the developing cortical plate. Gain- and loss-of-function approaches reveal that Nrg3 modulates the migration of interneurons into the cortical plate in a process that is dependent on the tyrosine kinase receptor ErbB4. Perturbation of Nrg3 signaling in conditional mutants leads to abnormal lamination of cortical interneurons. Nrg3 is therefore a critical mediator in the assembly of cortical inhibitory circuits.
Keywords: Erbb4; GABA; cerebral cortex; cortical plate; inhibition; interneuron; lamination; migration; neuregulins; pyramidal cell.
Copyright © 2017 The Authors. Published by Elsevier Inc. All rights reserved.
Figures





References
-
- Abe P., Mueller W., Schütz D., MacKay F., Thelen M., Zhang P., Stumm R. CXCR7 prevents excessive CXCL12-mediated downregulation of CXCR4 in migrating cortical interneurons. Development. 2014;141:1857–1863. - PubMed
-
- Anderson S.A., Eisenstat D.D., Shi L., Rubenstein J.L.R. Interneuron migration from basal forebrain to neocortex: dependence on Dlx genes. Science. 1997;278:474–476. - PubMed
-
- Aron A.R., Poldrack R.A. The cognitive neuroscience of response inhibition: relevance for genetic research in attention-deficit/hyperactivity disorder. Biol. Psychiatry. 2005;57:1285–1292. - PubMed
-
- Baudoin J.P., Viou L., Launay P.S., Luccardini C., Espeso Gil S., Kiyasova V., Irinopoulou T., Alvarez C., Rio J.P., Boudier T. Tangentially migrating neurons assemble a primary cilium that promotes their reorientation to the cortical plate. Neuron. 2012;76:1108–1122. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

