Colorectal cancer cells suppress CD4+ T cells immunity through canonical Wnt signaling
- PMID: 28147310
- PMCID: PMC5362476
- DOI: 10.18632/oncotarget.14834
Colorectal cancer cells suppress CD4+ T cells immunity through canonical Wnt signaling
Abstract
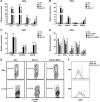
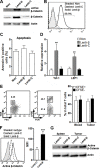
Understanding how colorectal cancer escapes from immunosurveillance and immune attack is important for developing novel immunotherapies for colorectal cancer. In this study we evaluated the role of canonical Wnt signaling in the regulation of T cell function in a mouse colorectal cancer model. We found that colorectal cancer cells expressed abundant Wnt ligands, and intratumoral T cells expressed various Frizzled proteins. Meanwhile, both active β-catenin and total β-catenin were elevated in intratumoral T cells. In vitro study indicated that colorectal cancer cells suppressed IFN-γ expression and increased IL-17a expression in activated CD4+ T cells. However, the cytotoxic activity of CD8+ T cells was not altered by colorectal cancer cells. To further evaluate the importance of Wnt signaling for CD4+ T cell-mediated cancer immunity, β-catenin expression was enforced in CD4+ T cells using lentiviral transduction. In an adoptive transfer model, enforced expression of β-catenin in intratumoral CD4+ T cells increased IL-17a expression, enhanced proliferation and inhibited apoptosis of colorectal cancer cells. Taken together, our study disclosed a new mechanism by which colorectal cancer impairs T cell immunity.
Keywords: CD4+ T cells immunity; canonical Wnt signaling; colorectal cancer; negatively regulate.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures





Similar articles
-
MicroRNA-1229 overexpression promotes cell proliferation and tumorigenicity and activates Wnt/β-catenin signaling in breast cancer.Oncotarget. 2016 Apr 26;7(17):24076-87. doi: 10.18632/oncotarget.8119. Oncotarget. 2016. PMID: 26992223 Free PMC article.
-
TTPAL Promotes Colorectal Tumorigenesis by Stabilizing TRIP6 to Activate Wnt/β-Catenin Signaling.Cancer Res. 2019 Jul 1;79(13):3332-3346. doi: 10.1158/0008-5472.CAN-18-2986. Epub 2019 Apr 24. Cancer Res. 2019. PMID: 31018940
-
Histone demethylase JMJD1A promotes colorectal cancer growth and metastasis by enhancing Wnt/β-catenin signaling.J Biol Chem. 2018 Jul 6;293(27):10606-10619. doi: 10.1074/jbc.RA118.001730. Epub 2018 May 25. J Biol Chem. 2018. PMID: 29802196 Free PMC article.
-
Therapeutic Potential of Targeting Wnt/β-Catenin Pathway in Treatment of Colorectal Cancer: Rational and Progress.J Cell Biochem. 2017 Aug;118(8):1979-1983. doi: 10.1002/jcb.25903. Epub 2017 Apr 25. J Cell Biochem. 2017. PMID: 28109136 Review.
-
Vitamin D and the regulation of Wnt/beta-catenin signaling and innate immunity in colorectal cancer.Nutr Rev. 2007 Aug;65(8 Pt 2):S118-20. doi: 10.1111/j.1753-4887.2007.tb00337.x. Nutr Rev. 2007. PMID: 17867386 Review. No abstract available.
Cited by
-
It's high-time to re-evaluate the value of induced-chemotherapy for reinforcing immunotherapy in colorectal cancer.Front Immunol. 2023 Oct 18;14:1241208. doi: 10.3389/fimmu.2023.1241208. eCollection 2023. Front Immunol. 2023. PMID: 37920463 Free PMC article. Review.
-
Prognostic and predictive role of immune microenvironment in colorectal cancer.World J Gastrointest Oncol. 2024 Mar 15;16(3):643-652. doi: 10.4251/wjgo.v16.i3.643. World J Gastrointest Oncol. 2024. PMID: 38577454 Free PMC article. Review.
-
Immune-Related Biomarkers Associated with Lung Metastasis from the Colorectal Cancer Microenvironment.J Interferon Cytokine Res. 2022 May;42(5):220-234. doi: 10.1089/jir.2021.0214. J Interferon Cytokine Res. 2022. PMID: 35576491 Free PMC article.
-
Dickkopf-related protein 3 alters aerobic glycolysis in pancreatic cancer BxPC-3 cells, promoting CD4+ T-cell activation and function.Eur J Med Res. 2021 Aug 14;26(1):93. doi: 10.1186/s40001-021-00567-x. Eur J Med Res. 2021. PMID: 34391478 Free PMC article.
-
Transcriptomic Analyses of the Adenoma-Carcinoma Sequence Identify Hallmarks Associated With the Onset of Colorectal Cancer.Front Oncol. 2021 Aug 11;11:704531. doi: 10.3389/fonc.2021.704531. eCollection 2021. Front Oncol. 2021. PMID: 34458146 Free PMC article.
References
-
- Prall F, Duhrkop T, Weirich V, Ostwald C, Lenz P, Nizze H, Barten M. Prognostic role of CD8+ tumor-infiltrating lymphocytes in stage III colorectal cancer with and without microsatellite instability. Hum Pathol. 2004;35:808–816. - PubMed
-
- Koch M, Beckhove P, J Op den Winkel, Autenrieth D, Wagner P, Nummer D, Specht S, Antolovic D, Galindo L, Schmitz-Winnenthal FH, Schirrmacher V, Buchler MW, Weitz J. Tumor infiltrating T lymphocytes in colorectal cancer: Tumor-selective activation and cytotoxic activity in situ. Ann Surg. 2006;244:986–992. discussion 992–983. - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

