Dclk1, a tumor stem cell marker, regulates pro-survival signaling and self-renewal of intestinal tumor cells
- PMID: 28148261
- PMCID: PMC5286867
- DOI: 10.1186/s12943-017-0594-y
Dclk1, a tumor stem cell marker, regulates pro-survival signaling and self-renewal of intestinal tumor cells
Retraction in
-
Retraction Note: Dclk1, a tumor stem cell marker, regulates pro-survival signaling and self-renewal of intestinal tumor cells.Mol Cancer. 2025 Apr 19;24(1):120. doi: 10.1186/s12943-025-02329-3. Mol Cancer. 2025. PMID: 40253332 Free PMC article. No abstract available.
Abstract
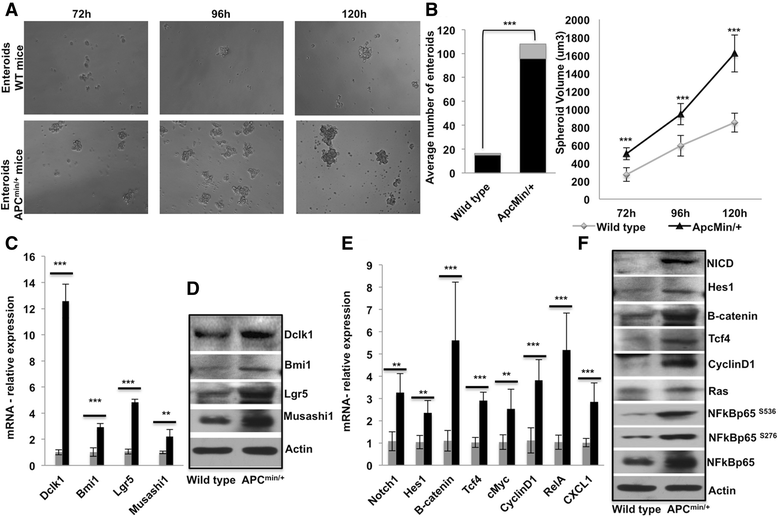
Background: More than 80% of intestinal neoplasia is associated with the adenomatous polyposis coli (APC) mutation. Doublecortin-like kinase 1 (Dclk1), a kinase protein, is overexpressed in colorectal cancer and specifically marks tumor stem cells (TSCs) that self-renew and increased the tumor progeny in Apc Min/+ mice. However, the role of Dclk1 expression and its contribution to regulating pro-survival signaling for tumor progression in Apc mutant cancer is poorly understood.
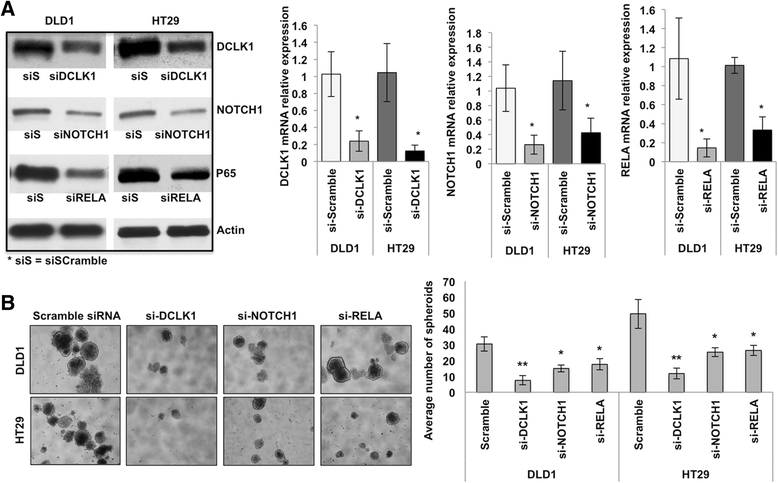
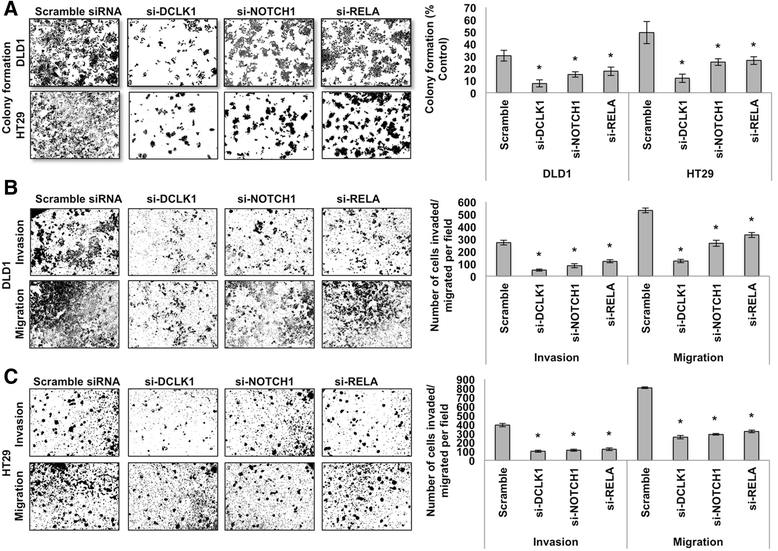
Methods: We analyzed DCLK1 and pro-survival signaling gene expression datasets of 329 specimens from TCGA Colon Adenocarcinoma Cancer Data. The network of DCLK1 and pro-survival signaling was analyzed utilizing the GeneMANIA database. We examined the expression levels of Dclk1 and other stem cell-associated markers, pro-survival signaling pathways, cell self-renewal in the isolated intestinal epithelial cells of Apc Min/+ mice with high-grade dysplasia and adenocarcinoma. To determine the functional role of Dclk1 for tumor progression, we knocked down Dclk1 and determined the pro-survival signaling pathways and stemness. We used siRNA technology to gene silence pro-survival signaling in colon cancer cells in vitro. We utilized FACS, IHC, western blot, RT-PCR, and clonogenic (self-renewal) assays.
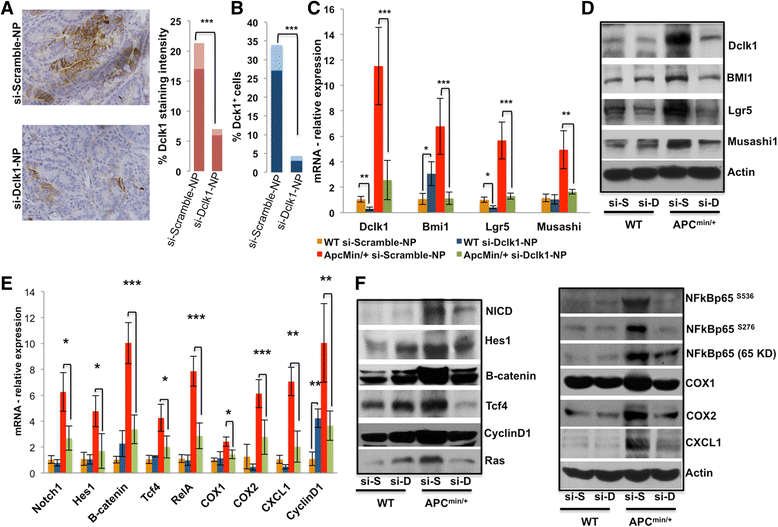
Results: We found a correlation between DCLK1 and pro-survival signaling expression. The expression of Dclk1 and stem cell-associated markers Lgr5, Bmi1, and Musashi1 were significantly higher in the intestinal epithelial cells of Apc Min/+ mice than in wild-type controls. Intestinal epithelial cells of Apc Min/+ mice showed increased expression of pro-survival signaling, pluripotency and self-renewal ability. Furthermore, the enteroids formed from the intestinal Dclk1+ cells of Apc Min/+ mice display higher pluripotency and pro-survival signaling. Dclk1 knockdown in Apc Min/+ mice attenuates intestinal adenomas and adenocarcinoma, and decreases pro-survival signaling and self-renewal. Knocking down RELA and NOTCH1 pro-survival signaling and DCLK1 in HT29 and DLD1 colon cancer cells in vitro reduced the tumor cells' ability to self-renew and survive.
Conclusion: Our results indicate that Dclk1 is essential in advancing intestinal tumorigenesis. Knocking down Dclk1 decreases tumor stemness and progression and is thus predicted to regulate pro-survival signaling and tumor cell pluripotency. This study provides a strong rationale to target Dclk1 as a treatment strategy for colorectal cancer.
Keywords: APC mutation; Cancer stem cells; Colorectal cancer; Dclk1; Intestinal epithelial cells; Nanoparticles; Pro-survival signaling; Self-renewal.
Figures



References
-
- Lewis A, Segditsas S, Deheragoda M, Pollard P, Jeffery R, Nye E, Lockstone H, Davis H, Clark S, Stamp G, et al. Severe polyposis in Apc(1322 T) mice is associated with submaximal Wnt signalling and increased expression of the stem cell marker Lgr5. Gut. 2010;59:1680–1686. doi: 10.1136/gut.2009.193680. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

