A longitudinal study of fecal calprotectin and the development of inflammatory bowel disease in ankylosing spondylitis
- PMID: 28148281
- PMCID: PMC5289027
- DOI: 10.1186/s13075-017-1223-2
A longitudinal study of fecal calprotectin and the development of inflammatory bowel disease in ankylosing spondylitis
Abstract
Background: Patients with ankylosing spondylitis (AS) are at increased risk of developing inflammatory bowel disease (IBD). We aimed to determine the variation in fecal calprotectin in AS over 5 years in relation to disease activity and medication and also to study the incidence of and predictors for development of IBD.
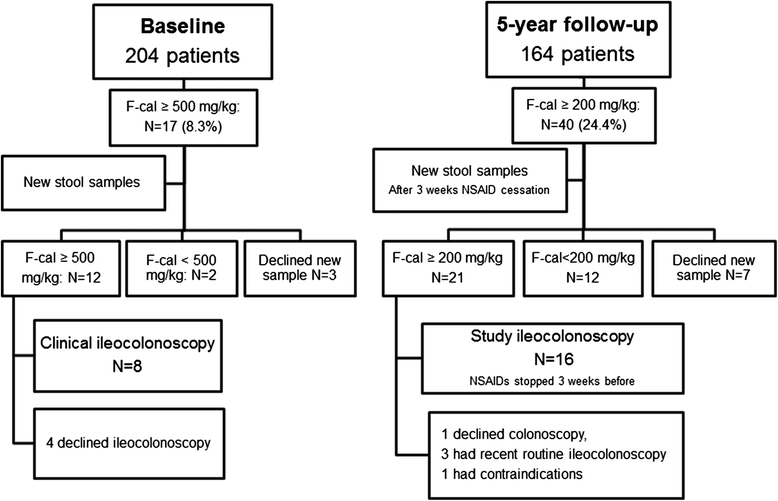
Methods: Fecal calprotectin was assessed at baseline (n = 204) and at 5-year follow-up (n = 164). The patients answered questionnaires and underwent clinical evaluations. At baseline and at 5-year follow-up, ileocolonoscopy was performed in patients with fecal calprotectin ≥500 mg/kg and ≥200 mg/kg, respectively. The medical records were checked for diagnoses of IBD during the follow-up period.
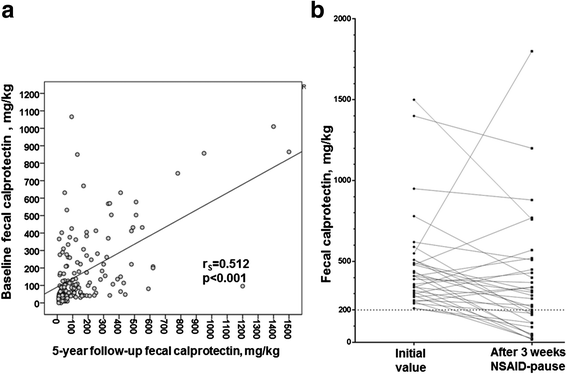
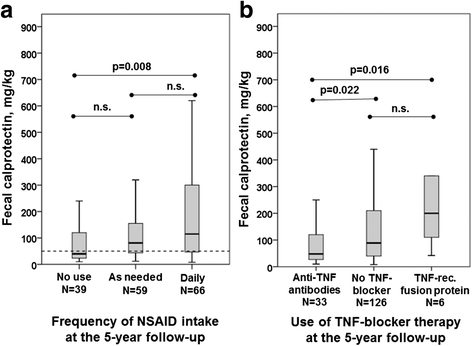
Results: Fecal calprotectin >50 mg/kg was found in two-thirds of the patients at both study visits. In 80% of the patients, fecal calprotectin changed by <200 mg/kg between the two measuring points. Baseline fecal calprotectin was positively correlated with Ankylosing Spondylitis Disease Activity Score based on C-reactive protein, Bath Ankylosing Spondylitis Disease Activity Index, Bath Ankylosing Spondylitis Functional Index, C-reactive protein, erythrocyte sedimentation rate, and fecal calprotectin at 5-year follow-up. The use of nonsteroidal anti-inflammatory drugs (NSAIDs) was associated with higher fecal calprotectin, and 3-week cessation of NSAIDs resulted in a drop of a median 116 mg/kg in fecal calprotectin. The use of tumor necrosis factor (TNF) blockers was associated with lower fecal calprotectin at both visits, but the users of TNF receptor fusion proteins had significantly higher fecal calprotectin than users of anti-TNF antibodies at 5-year follow-up. The 5-year incidence of Crohn's disease (CD) was 1.5% and was predicted by high fecal calprotectin.
Conclusions: Fecal calprotectin was elevated in a majority of the patients and was associated with disease activity and medication at both visits. CD developed in 1.5% of the patients with AS, and a high fecal calprotectin was the main predictor thereof. The results support a link between inflammation in the gut and the musculoskeletal system in AS. We propose that fecal calprotectin may be a potential biomarker to identify patients with AS at risk of developing IBD.
Trial registration: ClinicalTrials.gov identifier: NCT00858819 . Registered 9 March 2009. Last updated 28 May 2015.
Keywords: Ankylosing spondylitis; Crohn’s disease; Fecal calprotectin; Inflammatory bowel disease; Intestinal inflammation; Spondylarthritis; Ulcerative colitis.
Figures
Similar articles
-
A distinct gut microbiota composition in patients with ankylosing spondylitis is associated with increased levels of fecal calprotectin.Arthritis Res Ther. 2019 Nov 27;21(1):248. doi: 10.1186/s13075-019-2018-4. Arthritis Res Ther. 2019. PMID: 31771630 Free PMC article.
-
Calprotectin in ankylosing spondylitis--frequently elevated in feces, but normal in serum.Scand J Gastroenterol. 2012 Apr;47(4):435-44. doi: 10.3109/00365521.2011.648953. Epub 2012 Jan 10. Scand J Gastroenterol. 2012. PMID: 22229862
-
Serum calprotectin as a biomarker for Crohn's disease.J Crohns Colitis. 2013 Dec;7(12):e678-83. doi: 10.1016/j.crohns.2013.06.008. Epub 2013 Jul 9. J Crohns Colitis. 2013. PMID: 23845231 Clinical Trial.
-
[Fecal Calprotectin in Inflammatory Bowel Disease].Korean J Gastroenterol. 2016 May 25;67(5):233-7. doi: 10.4166/kjg.2016.67.5.233. Korean J Gastroenterol. 2016. PMID: 27206433 Review. Korean.
-
Faecal Calprotectin for the Diagnosis of Bowel Inflammation in Patients With Rheumatological Diseases: A Systematic Review.J Crohns Colitis. 2020 Jun 19;14(5):688-693. doi: 10.1093/ecco-jcc/jjz205. J Crohns Colitis. 2020. PMID: 31858121
Cited by
-
Characteristics of the intestinal microbiome in ankylosing spondylitis.Exp Ther Med. 2021 Jul;22(1):676. doi: 10.3892/etm.2021.10108. Epub 2021 Apr 24. Exp Ther Med. 2021. PMID: 33986841 Free PMC article.
-
Expression of CD44 in Leukocyte Subpopulations in Patients with Inflammatory Bowel Diseases.Diagnostics (Basel). 2022 Aug 20;12(8):2014. doi: 10.3390/diagnostics12082014. Diagnostics (Basel). 2022. PMID: 36010364 Free PMC article.
-
Fecal Calprotectin, CRP and Leucocytes in IBD Patients: Comparison of Biomarkers With Biopsy Results.J Can Assoc Gastroenterol. 2020 Mar 27;4(2):84-90. doi: 10.1093/jcag/gwaa009. eCollection 2021 Apr. J Can Assoc Gastroenterol. 2020. PMID: 33855266 Free PMC article.
-
Risk for development of inflammatory bowel disease under inhibition of interleukin 17: A systematic review and meta-analysis.PLoS One. 2020 May 27;15(5):e0233781. doi: 10.1371/journal.pone.0233781. eCollection 2020. PLoS One. 2020. PMID: 32459816 Free PMC article.
-
GlutenSpA trial: protocol for a randomised double-blind placebo-controlled trial of the impact of a gluten-free diet on quality of life in patients with axial spondyloarthritis.BMJ Open. 2020 Nov 20;10(11):e038715. doi: 10.1136/bmjopen-2020-038715. BMJ Open. 2020. PMID: 33444189 Free PMC article. Clinical Trial.
References
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

