Variation in human dental pulp stem cell ageing profiles reflect contrasting proliferative and regenerative capabilities
- PMID: 28148303
- PMCID: PMC5288874
- DOI: 10.1186/s12860-017-0128-x
Variation in human dental pulp stem cell ageing profiles reflect contrasting proliferative and regenerative capabilities
Abstract
Background: Dental pulp stem cells (DPSCs) are increasingly being recognized as a viable cell source for regenerative medicine. Although significant variations in their ex vivo expansion are well-established, DPSC proliferative heterogeneity remains poorly understood, despite such characteristics influencing their regenerative and therapeutic potential. This study assessed clonal human DPSC regenerative potential and the impact of cellular senescence on these responses, to better understand DPSC functional behaviour.
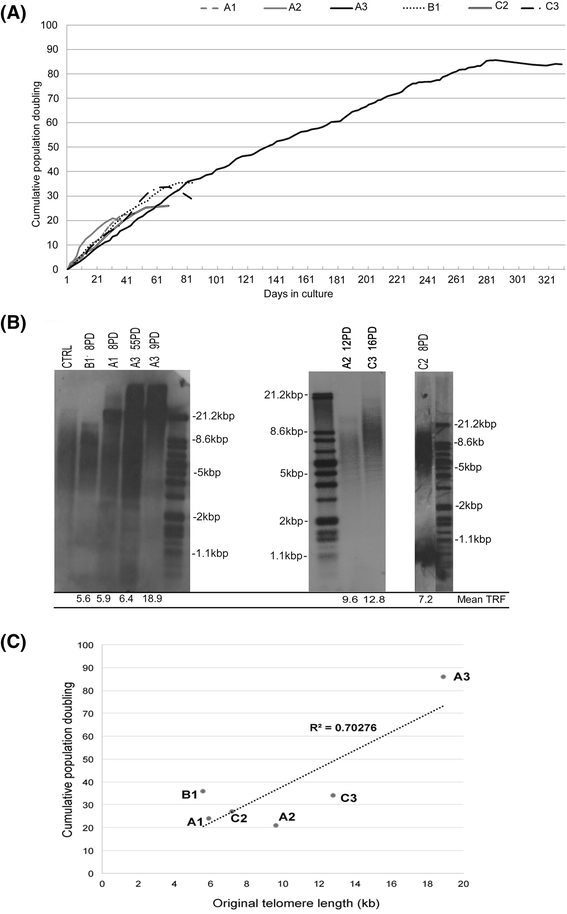
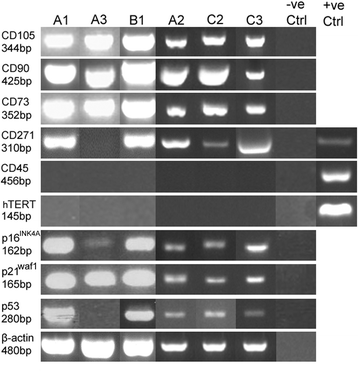
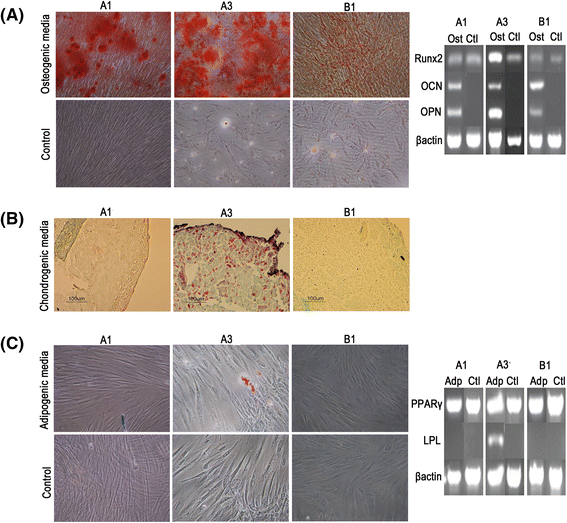
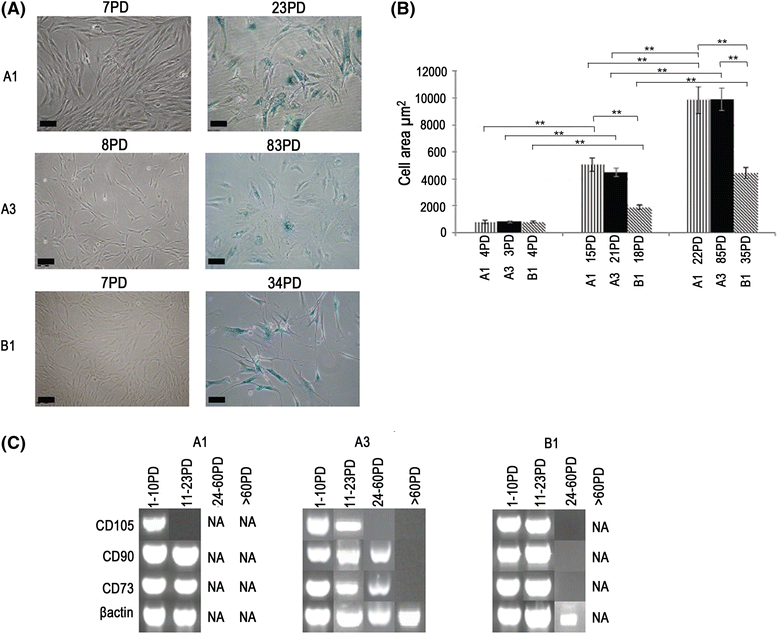
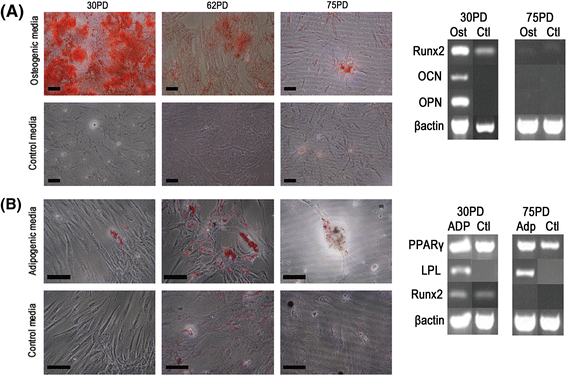
Results: All DPSCs were negative for hTERT. Whilst one DPSC population reached >80 PDs before senescence, other populations only achieved <40 PDs, correlating with DPSCs with high proliferative capacities possessing longer telomeres (18.9 kb) than less proliferative populations (5-13 kb). High proliferative capacity DPSCs exhibited prolonged stem cell marker expression, but lacked CD271. Early-onset senescence, stem cell marker loss and positive CD271 expression in DPSCs with low proliferative capacities were associated with impaired osteogenic and chondrogenic differentiation, favouring adipogenesis. DPSCs with high proliferative capacities only demonstrated impaired differentiation following prolonged expansion (>60 PDs).
Conclusions: This study has identified that proliferative and regenerative heterogeneity is related to contrasting telomere lengths and CD271 expression between DPSC populations. These characteristics may ultimately be used to selectively screen and isolate high proliferative capacity/multi-potent DPSCs for regenerative medicine exploitation.
Keywords: CD271; Cellular senescence; Cumulative population doublings; Dental pulp; Differentiation; Multi-potency; Stem cells; Telomeres.
Figures
Similar articles
-
Differential SOD2 and GSTZ1 profiles contribute to contrasting dental pulp stem cell susceptibilities to oxidative damage and premature senescence.Stem Cell Res Ther. 2021 Feb 17;12(1):142. doi: 10.1186/s13287-021-02209-9. Stem Cell Res Ther. 2021. PMID: 33596998 Free PMC article.
-
Evaluation of Dental Pulp Stem Cell Heterogeneity and Behaviour in 3D Type I Collagen Gels.Biomed Res Int. 2020 Sep 10;2020:3034727. doi: 10.1155/2020/3034727. eCollection 2020. Biomed Res Int. 2020. PMID: 32964026 Free PMC article.
-
Preliminary Evaluation of Proliferation, Wound Healing Properties, Osteogenic and Chondrogenic Potential of Dental Pulp Stem Cells Obtained from Healthy and Periodontitis Affected Teeth.Cells. 2021 Aug 18;10(8):2118. doi: 10.3390/cells10082118. Cells. 2021. PMID: 34440887 Free PMC article.
-
Senescence of dental pulp stem cells: phenotypes, underlying mechanisms and regulatory molecules.Hum Cell. 2025 Jul 11;38(5):127. doi: 10.1007/s13577-025-01259-y. Hum Cell. 2025. PMID: 40646329 Review.
-
Systematic Review of Human Dental Pulp Stem Cells for Cartilage Regeneration.Tissue Eng Part B Rev. 2020 Feb;26(1):1-12. doi: 10.1089/ten.TEB.2019.0140. Epub 2020 Jan 22. Tissue Eng Part B Rev. 2020. PMID: 31744404
Cited by
-
Dental stem cell-conditioned medium for tissue regeneration: Optimization of production and storage.World J Stem Cells. 2022 Apr 26;14(4):287-302. doi: 10.4252/wjsc.v14.i4.287. World J Stem Cells. 2022. PMID: 35662860 Free PMC article.
-
Low Molecular Weight Hyaluronic Acid Effect on Dental Pulp Stem Cells In Vitro.Biomolecules. 2020 Dec 28;11(1):22. doi: 10.3390/biom11010022. Biomolecules. 2020. PMID: 33379324 Free PMC article.
-
Comparative characterization and analysis of telomere length in stem cells derived from deciduous and permanent teeth.Dent Res J (Isfahan). 2022 Aug 16;19:64. eCollection 2022. Dent Res J (Isfahan). 2022. PMID: 36159052 Free PMC article.
-
Liposomal Delivery of Demineralized Dentin Matrix for Dental Tissue Regeneration.Tissue Eng Part A. 2018 Jul;24(13-14):1057-1065. doi: 10.1089/ten.TEA.2017.0419. Epub 2018 Feb 21. Tissue Eng Part A. 2018. PMID: 29316874 Free PMC article.
-
Effects of Cellular Senescence on Dental Follicle Cells.Pharmacology. 2021;106(3-4):137-142. doi: 10.1159/000510014. Epub 2020 Sep 25. Pharmacology. 2021. PMID: 32980839 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

