Characterisation of osteophytes as an autologous bone graft source: An experimental study in vivo and in vitro
- PMID: 28148490
- PMCID: PMC5331175
- DOI: 10.1302/2046-3758.62.BJR-2016-0199.R1
Characterisation of osteophytes as an autologous bone graft source: An experimental study in vivo and in vitro
Abstract
Objectives: Osteophytes are products of active endochondral and intramembranous ossification, and therefore could theoretically provide significant efficacy as bone grafts. In this study, we compared the bone mineralisation effectiveness of osteophytes and cancellous bone, including their effects on secretion of growth factors and anabolic effects on osteoblasts.
Methods: Osteophytes and cancellous bone obtained from human patients were transplanted onto the calvaria of severe combined immunodeficient mice, with Calcein administered intra-peritoneally for fluorescent labelling of bone mineralisation. Conditioned media were prepared using osteophytes and cancellous bone, and growth factor concentration and effects of each graft on proliferation, differentiation and migration of osteoblastic cells were assessed using enzyme-linked immunosorbent assays, MTS ((3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium)) assays, quantitative real-time polymerase chain reaction, and migration assays.
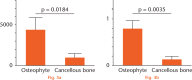
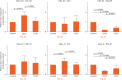
Results: After six weeks, the area of mineralisation was significantly higher for the transplanted osteophytes than for the cancellous bone (43803 μm2, sd 14660 versus 9421 μm2, sd 5032, p = 0.0184, one-way analysis of variance). Compared with cancellous bone, the conditioned medium prepared using osteophytes contained a significantly higher amounts of transforming growth factor (TGF)-β1 (471 pg/ml versus 333 pg/ml, p = 0.0001, Wilcoxon rank sum test), bone morphogenetic protein (BMP)-2 (47.75 pg/ml versus 32 pg/ml, p = 0.0214, Wilcoxon rank sum test) and insulin-like growth factor (IGF)-1 (314.5 pg/ml versus 191 pg/ml, p = 0.0418, Wilcoxon rank sum test). The stronger effects of osteophytes towards osteoblasts in terms of a higher proliferation rate, upregulation of gene expression of differentiation markers such as alpha-1 type-1 collagen and alkaline phosphate, and higher migration, compared with cancellous bone, was confirmed.
Conclusion: We provide evidence of favourable features of osteophytes for bone mineralisation through a direct effect on osteoblasts. The acceleration in metabolic activity of the osteophyte provides justification for future studies evaluating the clinical use of osteophytes as autologous bone grafts.Cite this article: K. Ishihara, K. Okazaki, T. Akiyama, Y. Akasaki, Y. Nakashima. Characterisation of osteophytes as an autologous bone graft source: An experimental study in vivo and in vitro. Bone Joint Res 2017;6:73-81. DOI: 10.1302/2046-3758.62.BJR-2016-0199.R1.
Keywords: Bone graft; Growth factor; Osteophyte; Osteotomy.
© 2017 Okazaki et al.
Conflict of interest statement
Figures





References
-
- Sen MK, Miclau T. Autologous iliac crest bone graft: should it still be the gold standard for treating nonunions? Injury 2007;38:S75-S80. - PubMed
-
- Fillingham Y, Jacobs J. Bone grafts and their substitutes. Bone Joint J 2016;98-B(1 Suppl A):6-9. - PubMed
-
- Younger EM, Chapman MW. Morbidity at bone graft donor sites. J Orthop Trauma 1989;3:192-195. - PubMed
-
- Gelse K, Söder S, Eger W, et al. Osteophyte development–molecular characterization of differentiation stages. Osteoarthritis Cartilage 2003;11:141-148. - PubMed
-
- van der Kraan PM, van den Berg WB. Osteophytes: relevance and biology. Osteoarthritis Cartilage 2007;15:237-244. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

