High-dose immunosuppressive therapy and autologous HCT for relapsing-remitting MS
- PMID: 28148635
- PMCID: PMC5331868
- DOI: 10.1212/WNL.0000000000003660
High-dose immunosuppressive therapy and autologous HCT for relapsing-remitting MS
Abstract
Objective: To evaluate the safety, efficacy, and durability of multiple sclerosis (MS) disease stabilization after high-dose immunosuppressive therapy (HDIT) and autologous hematopoietic cell transplantation (HCT).
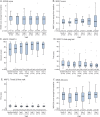
Methods: High-Dose Immunosuppression and Autologous Transplantation for Multiple Sclerosis (HALT-MS) is a phase II clinical trial of HDIT/HCT for patients with relapsing-remitting (RR) MS who experienced relapses with disability progression (Expanded Disability Status Scale [EDSS] 3.0-5.5) while on MS disease-modifying therapy. The primary endpoint was event-free survival (EFS), defined as survival without death or disease activity from any one of: disability progression, relapse, or new lesions on MRI. Participants were evaluated through 5 years posttransplant. Toxicities were reported using the National Cancer Institute Common Terminology Criteria for Adverse Events (AE).
Results: Twenty-five participants were evaluated for transplant and 24 participants underwent HDIT/HCT. Median follow-up was 62 months (range 12-72). EFS was 69.2% (90% confidence interval [CI] 50.2-82.1). Progression-free survival, clinical relapse-free survival, and MRI activity-free survival were 91.3% (90% CI 74.7%-97.2%), 86.9% (90% CI 69.5%-94.7%), and 86.3% (90% CI 68.1%-94.5%), respectively. AE due to HDIT/HCT were consistent with expected toxicities and there were no significant late neurologic adverse effects noted. Improvements were noted in neurologic disability with a median change in EDSS of -0.5 (interquartile range -1.5 to 0.0; p = 0.001) among participants who survived and completed the study.
Conclusion: HDIT/HCT without maintenance therapy was effective for inducing long-term sustained remissions of active RRMS at 5 years.
Clinicaltrialsgov identifier: NCT00288626.
Classification of evidence: This study provides Class IV evidence that participants with RRMS experienced sustained remissions with toxicities as expected from HDIT/HCT.
Copyright © 2017 The Author(s). Published by Wolters Kluwer Health, Inc. on behalf of the American Academy of Neurology.
Figures



References
-
- Sormani MP, Muraro PA, Saccardi R, Mancardi G. NEDA status in highly active MS can be more easily obtained with autologous hematopoietic stem cell transplantation than other drugs. Mult Scler Epub 2016 Apr 26. - PubMed
-
- Imitola J, Racke MK. Is no evidence of disease activity a realistic goal for patients with multiple sclerosis? JAMA Neurol 2015;72:145–147. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
