A phosphorus threshold for mycoheterotrophic plants in tropical forests
- PMID: 28148744
- PMCID: PMC5310599
- DOI: 10.1098/rspb.2016.2093
A phosphorus threshold for mycoheterotrophic plants in tropical forests
Erratum in
-
Correction to 'A phosphorus threshold for mycoheterotrophic plants in tropical forests'.Proc Biol Sci. 2017 Dec 20;284(1869):20172372. doi: 10.1098/rspb.2017.2372. Proc Biol Sci. 2017. PMID: 29237863 Free PMC article. No abstract available.
Abstract
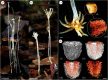
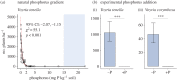
The majority of terrestrial plants associate with arbuscular mycorrhizal (AM) fungi, which typically facilitate the uptake of limiting mineral nutrients by plants in exchange for plant carbon. However, hundreds of non-photosynthetic plant species-mycoheterotrophs-depend entirely on AM fungi for carbon as well as mineral nutrition. Mycoheterotrophs can provide insight into the operation and regulation of AM fungal relationships, but little is known about the factors, fungal or otherwise, that affect mycoheterotroph abundance and distribution. In a lowland tropical forest in Panama, we conducted the first systematic investigation into the influence of abiotic factors on the abundance and distribution of mycoheterotrophs, to ask whether the availability of nitrogen and phosphorus altered the occurrence of mycoheterotrophs and their AM fungal partners. Across a natural fertility gradient spanning the isthmus of Panama, and also in a long-term nutrient-addition experiment, mycoheterotrophs were entirely absent when soil exchangeable phosphate concentrations exceeded 2 mg P kg-1 Experimental phosphorus addition reduced the abundance of AM fungi, and also reduced the abundance of the specific AM fungal taxa required by the mycoheterotrophs, suggesting that the phosphorus sensitivity of mycoheterotrophs is underpinned by the phosphorus sensitivity of their AM fungal hosts. The soil phosphorus concentration of 2 mg P kg-1 also corresponds to a marked shift in tree community composition and soil phosphatase activity across the fertility gradient, suggesting that our findings have broad ecological significance.
Keywords: arbuscular mycorrhizal fungi; epiparisitism; mycoheterotroph; phosphorus; tropical forest.
© 2017 The Author(s).
Figures


References
-
- Alexander I, Lee S. 2005. Mycorrhizas and ecosystem processes in tropical rain forest: implications for diversity. In Biotic interactions in the tropics (eds Burslem D, Pinard M, Hartley S), pp. 165–203. Cambridge, UK: Cambridge University Press.
-
- Jakobsen I, Rosendahl L. 1990. Carbon flow into soil and external hyphae from roots of mycorrhizal cucumber plants. New Phytol. 115, 77–83. (10.1111/j.1469-8137.1990.tb00924.x) - DOI
-
- Smith SE, Read DJ. 2008. Mycorrhizal symbiosis, 3rd edn New York, NY: Academic Press.
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources

