Immunization with Low Doses of Recombinant Postfusion or Prefusion Respiratory Syncytial Virus F Primes for Vaccine-Enhanced Disease in the Cotton Rat Model Independently of the Presence of a Th1-Biasing (GLA-SE) or Th2-Biasing (Alum) Adjuvant
- PMID: 28148790
- PMCID: PMC5375676
- DOI: 10.1128/JVI.02180-16
Immunization with Low Doses of Recombinant Postfusion or Prefusion Respiratory Syncytial Virus F Primes for Vaccine-Enhanced Disease in the Cotton Rat Model Independently of the Presence of a Th1-Biasing (GLA-SE) or Th2-Biasing (Alum) Adjuvant
Abstract
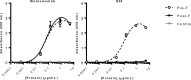
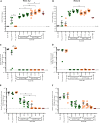
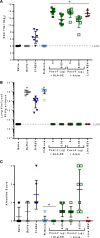
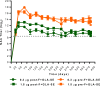
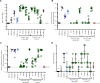
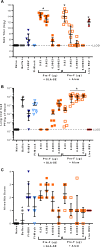
Respiratory syncytial virus (RSV) infection of children previously immunized with a nonlive, formalin-inactivated (FI)-RSV vaccine has been associated with serious enhanced respiratory disease (ERD). Consequently, detailed studies of potential ERD are a critical step in the development of nonlive RSV vaccines targeting RSV-naive children and infants. The fusion glycoprotein (F) of RSV in either its postfusion (post-F) or prefusion (pre-F) conformation is a target for neutralizing antibodies and therefore an attractive antigen candidate for a pediatric RSV subunit vaccine. Here, we report the evaluation of RSV post-F and pre-F in combination with glucopyranosyl lipid A (GLA) integrated into stable emulsion (SE) (GLA-SE) and alum adjuvants in the cotton rat model. Immunization with optimal doses of RSV F antigens in the presence of GLA-SE induced high titers of virus-neutralizing antibodies and conferred complete lung protection from virus challenge, with no ERD signs in the form of alveolitis. To mimic a waning immune response, and to assess priming for ERD under suboptimal conditions, an antigen dose de-escalation study was performed in the presence of either GLA-SE or alum. At low RSV F doses, alveolitis-associated histopathology was unexpectedly observed with either adjuvant at levels comparable to FI-RSV-immunized controls. This occurred despite neutralizing-antibody titers above the minimum levels required for protection and with no/low virus replication in the lungs. These results emphasize the need to investigate a pediatric RSV vaccine candidate carefully for priming of ERD over a wide dose range, even in the presence of strong neutralizing activity, Th1 bias-inducing adjuvant, and protection from virus replication in the lower respiratory tract.IMPORTANCE RSV disease is of great importance worldwide, with the highest burden of serious disease occurring upon primary infection in infants and children. FI-RSV-induced enhanced disease, observed in the 1960s, presented a major and ongoing obstacle for the development of nonlive RSV vaccine candidates. The findings presented here underscore the need to evaluate a nonlive RSV vaccine candidate during preclinical development over a wide dose range in the cotton rat RSV enhanced-disease model, as suboptimal dosing of several RSV F subunit vaccine candidates led to the priming for ERD. These observations are relevant to the validity of the cotton rat model itself and to safe development of nonlive RSV vaccines for seronegative infants and children.
Keywords: GLA-SE; RSV; RSV F; cotton rat; enhanced RSV disease; respiratory syncytial virus; vaccine.
Copyright © 2017 American Society for Microbiology.
Figures


References
-
- Falloon J, Ji F, Curtis C, Bart S, Sheldon E, Krieger D, Dubovsky F, Lambert S, Takas T, Villafana T, Esser MT. 2016. A phase 1a, first-in-human, randomized study of a respiratory syncytial virus F protein vaccine with and without a Toll-like receptor-4 agonist and stable emulsion adjuvant. Vaccine 34:2847–2854. doi:10.1016/j.vaccine.2016.04.002. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

