HIV-1 Vpu Downmodulates ICAM-1 Expression, Resulting in Decreased Killing of Infected CD4+ T Cells by NK Cells
- PMID: 28148794
- PMCID: PMC5375687
- DOI: 10.1128/JVI.02442-16
HIV-1 Vpu Downmodulates ICAM-1 Expression, Resulting in Decreased Killing of Infected CD4+ T Cells by NK Cells
Abstract
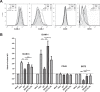
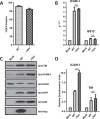
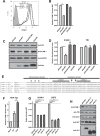
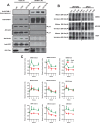
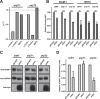
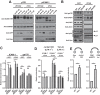
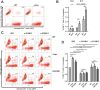
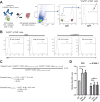
HIV-1 Vpu is known to alter the expression of numerous cell surface molecules. Given the ever-increasing list of Vpu targets identified to date, we undertook a proteomic screen to discover novel cell membrane proteins modulated by this viral protein. Plasma membrane proteome isolates from Vpu-inducible T cells were subjected to stable isotope labeling of amino acids in cell culture (SILAC)-based mass spectrometry analysis, and putative targets were validated by infection of primary CD4+ T cells. We report here that while intercellular adhesion molecule 1 (ICAM-1) and ICAM-3 are upregulated by HIV-1 infection, expression of Vpu offsets this increase by downregulating these molecules from the cell surface. Specifically, we show that Vpu is sufficient to downregulate and deplete ICAM-1 in a manner requiring the Vpu transmembrane domain and a dual-serine (S52/S56) motif necessary for recruitment of the beta-transducin repeat-containing E3 ubiquitin protein ligase (β-TrCP) component of the Skp, Cullin, F-box (SCFβ-TrCP) E3 ubiquitin ligase. Vpu interacts with ICAM-1 to induce its proteasomal degradation. Interestingly, the E3 ubiquitin ligase component β-TrCP-1 is dispensable for ICAM-1 surface downregulation yet is necessary for ICAM-1 degradation. Functionally, Vpu-mediated ICAM-1 downregulation lowers packaging of this adhesion molecule into virions, resulting in decreased infectivity. Importantly, while Vpu-mediated downregulation of ICAM-3 has a limited effect on the conjugation of NK cells to HIV-1-infected CD4+ T cells, downregulation of ICAM-1 by Vpu results in a reduced ability of NK cells to bind and kill infected T cells. Vpu-mediated ICAM-1 downregulation may therefore represent an evolutionary compromise in viral fitness by impeding the formation of cell-to-cell contacts between immune cells and infected T cells at the cost of decreased virion infectivity.IMPORTANCE The major barrier to eradicating HIV-1 infection is the establishment of treatment-resistant reservoirs early in infection. Vpu-mediated ICAM-1 downregulation may contribute to the evasion of cell-mediated immunity during acute infection to promote viral dissemination and the development of viral reservoirs. By aiding the immune system to clear infection prior to the development of reservoirs, novel treatments designed to disrupt Vpu-mediated ICAM-1 downregulation may be beneficial during acute infection or as a prophylactic treatment.
Keywords: ICAM-1; NK cell-mediated killing; Vpu; human immunodeficiency virus; immune evasion; immunological synapse; viral infectivity.
Copyright © 2017 American Society for Microbiology.
Figures

Similar articles
-
HIV-1 Vpu Promotes Phagocytosis of Infected CD4+ T Cells by Macrophages through Downregulation of CD47.mBio. 2021 Aug 31;12(4):e0192021. doi: 10.1128/mBio.01920-21. Epub 2021 Aug 24. mBio. 2021. PMID: 34425695 Free PMC article.
-
β-TrCP is dispensable for Vpu's ability to overcome the CD317/Tetherin-imposed restriction to HIV-1 release.Retrovirology. 2011 Feb 10;8:9. doi: 10.1186/1742-4690-8-9. Retrovirology. 2011. PMID: 21310048 Free PMC article.
-
HIV-1 Nef and Vpu Interfere with L-Selectin (CD62L) Cell Surface Expression To Inhibit Adhesion and Signaling in Infected CD4+ T Lymphocytes.J Virol. 2015 May;89(10):5687-700. doi: 10.1128/JVI.00611-15. Epub 2015 Mar 11. J Virol. 2015. PMID: 25822027 Free PMC article.
-
β-TrCP dependency of HIV-1 Vpu-induced downregulation of CD4 and BST-2/tetherin.Curr HIV Res. 2012 Jun;10(4):307-14. doi: 10.2174/157016212800792441. Curr HIV Res. 2012. PMID: 22524179 Review.
-
Interactions of viral protein U (Vpu) with cellular factors.Curr Top Microbiol Immunol. 2009;339:27-45. doi: 10.1007/978-3-642-02175-6_2. Curr Top Microbiol Immunol. 2009. PMID: 20012522 Review.
Cited by
-
Proteasomal Degradation Machinery: Favorite Target of HIV-1 Proteins.Front Microbiol. 2018 Nov 21;9:2738. doi: 10.3389/fmicb.2018.02738. eCollection 2018. Front Microbiol. 2018. PMID: 30524389 Free PMC article. Review.
-
Large-Scale Arrayed Analysis of Protein Degradation Reveals Cellular Targets for HIV-1 Vpu.Cell Rep. 2018 Feb 27;22(9):2493-2503. doi: 10.1016/j.celrep.2018.01.091. Cell Rep. 2018. PMID: 29490283 Free PMC article.
-
Negative Regulation and Protective Function of Natural Killer Cells in HIV Infection: Two Sides of a Coin.Front Immunol. 2022 Mar 7;13:842831. doi: 10.3389/fimmu.2022.842831. eCollection 2022. Front Immunol. 2022. PMID: 35320945 Free PMC article. Review.
-
Beyond Channel Activity: Protein-Protein Interactions Involving Viroporins.Subcell Biochem. 2018;88:329-377. doi: 10.1007/978-981-10-8456-0_15. Subcell Biochem. 2018. PMID: 29900504 Free PMC article. Review.
-
HIV-1 Vpu Promotes Phagocytosis of Infected CD4+ T Cells by Macrophages through Downregulation of CD47.mBio. 2021 Aug 31;12(4):e0192021. doi: 10.1128/mBio.01920-21. Epub 2021 Aug 24. mBio. 2021. PMID: 34425695 Free PMC article.
References
-
- Salazar-Gonzalez JF, Salazar MG, Keele BF, Learn GH, Giorgi EE, Li H, Decker JM, Wang S, Baalwa J, Kraus MH, Parrish NF, Shaw KS, Guffey MB, Bar KJ, Davis KL, Ochsenbauer-Jambor C, Kappes JC, Saag MS, Cohen MS, Mulenga J, Derdeyn CA, Allen S, Hunter E, Markowitz M, Hraber P, Perelson AS, Bhattacharya T, Haynes BF, Korber BT, Hahn BH, Shaw GM. 2009. Genetic identity, biological phenotype, and evolutionary pathways of transmitted/founder viruses in acute and early HIV-1 infection. J Exp Med 206:1273–1289. doi:10.1084/jem.20090378. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

