Therapy with CTLA4-Ig and an antiviral monoclonal antibody controls chikungunya virus arthritis
- PMID: 28148840
- PMCID: PMC5448557
- DOI: 10.1126/scitranslmed.aah3438
Therapy with CTLA4-Ig and an antiviral monoclonal antibody controls chikungunya virus arthritis
Abstract
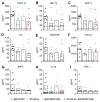
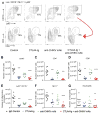
In 2013, chikungunya virus (CHIKV) transmission was documented in the Western Hemisphere, and the virus has since spread throughout the Americas with more than 1.8 million people infected in more than 40 countries. CHIKV targets the joints, resulting in symmetric polyarthritis that clinically mimics rheumatoid arthritis and can endure for months to years. At present, no approved treatment is effective in preventing or controlling CHIKV infection or disease. We treated mice with eight different disease-modifying antirheumatic drugs and identified CLTA4-Ig (abatacept) and tofacitinib as candidate therapies based on their ability to decrease acute joint swelling. CTLA4-Ig reduced T cell accumulation in the joints of infected animals without affecting viral infection. Whereas monotherapy with CTLA4-Ig or a neutralizing anti-CHIKV human monoclonal antibody provided partial clinical improvement, therapy with both abolished swelling and markedly reduced levels of chemokines, proinflammatory cytokines, and infiltrating leukocytes. Thus, combination CTLA4-Ig and antiviral antibody therapy controls acute CHIKV infection and arthritis and may be a candidate for testing in humans.
Copyright © 2017, American Association for the Advancement of Science.
Conflict of interest statement
Figures





Comment in
-
Blunting CHIKV infection by keeping T cells in check.Sci Transl Med. 2017 Feb 1;9(375):eaam6567. doi: 10.1126/scitranslmed.aam6567. Sci Transl Med. 2017. PMID: 28148847
-
Acute inflammatory arthritis: Potential therapies for chikungunya arthritis.Nat Rev Rheumatol. 2017 Apr;13(4):196. doi: 10.1038/nrrheum.2017.21. Epub 2017 Feb 16. Nat Rev Rheumatol. 2017. PMID: 28202915 No abstract available.
References
-
- Rodriguez-Morales AJ, Gil-Restrepo AF, Ramírez-Jaramillo V, Montoya-Arias CP, Acevedo-Mendoza WF, Bedoya-Arias JE, Chica-Quintero LA, Murillo-García DR, García-Robledo JE, Castrillón-Spitia JD, Londoño JJ, Bedoya-Rendón HD, Cárdenas-Pérez JJ, Cardona-Ospina JA, Lagos-Grisales GJ. Post-chikungunya chronic inflammatory rheumatism: Results from a retrospective follow-up study of 283 adult and child cases in La Virginia, Risaralda, Colombia. F1000Res. 2016;5:360. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

