Palmdelphin promotes myoblast differentiation and muscle regeneration
- PMID: 28148961
- PMCID: PMC5288731
- DOI: 10.1038/srep41608
Palmdelphin promotes myoblast differentiation and muscle regeneration
Abstract
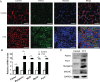
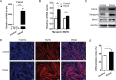
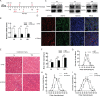
Differentiation of myoblasts is essential in the development and regeneration of skeletal muscles to form multinucleated, contractile muscle fibers. However, the process of myoblast differentiation in mammals is complicated and requires to be further investigated. In this study, we found Palmdelphin (Palmd), a cytosolic protein, promotes myoblast differentiation. Palmd is predominantly expressed in the cytosol of myoblasts and is gradually up-regulated after differentiation. Knockdown of Palmd by small interfering RNA (siRNA) in C2C12 markedly inhibits myogenic differentiation, suggesting a specific role of Palmd in the morphological changes of myoblast differentiation program. Overexpression of Palmd in C2C12 enhances myogenic differentiation. Remarkably, inhibition of Palmd results in impaired myotube formation during muscle regeneration after injury. These findings reveal a new cytosolic protein that promotes mammalian myoblast differentiation and provide new insights into the molecular regulation of muscle formation.
Conflict of interest statement
The authors declare no competing financial interests.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

