Endometrial and Ovarian Cancer with MR Imaging Importance of Serum HE4 and CA 125 Levels in the Extent of Disease at Evaluation
- PMID: 28149145
- PMCID: PMC5268602
- DOI: 10.5152/eurasianjmed.2016.0259
Endometrial and Ovarian Cancer with MR Imaging Importance of Serum HE4 and CA 125 Levels in the Extent of Disease at Evaluation
Abstract
Objective: Currently, no clinically useful tumor marker is available for primary diagnosis in endometrial cancer. Human epididymis protein-4 (HE-4) has high sensitivity and specificity as a tumor marker. Further, HE-4 has been shown to be elevated in early stage endometrial cancer and is more sensitive than CA 125. In our study, CA 125 and HE-4 reputation as a tumor marker for diagnosis of ovarian and endometrial cancer with the use of both the availability and affect the way we investigated the rate of diagnosis.
Materials and methods: Here 20 patients with ovarian cancer, 26 patients with endometrial cancer, which had been histologically diagnosed, and 40 healthy volunteers were included. Peripheral blood samples were taken and serum CA 125 and HE-4 were tested.
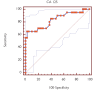
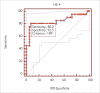
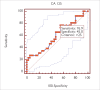
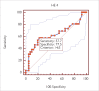
Results: Serum CA 125 and HE-4 levels in patients with ovarian cancer were found to be significantly higher than those in healthy volunteers (p<0.05). Receiver-operating characteristic (ROC) analysis was performed. For patients with ovarian cancer and healthy controls, the CA 125 (0.83) and HE-4 (0.84) levels showed increased sensitivity (95%). There was no significant difference in the CA 125 levels in patients with endometrial cancer and healthy controls (p>0.05), whereas HE-4 levels were found to be higher in patients with endometrial cancer than in healthy controls (p<0.05). ROC analysis was performed. For endometrial cancer patients and healthy controls, the CA 125 (0.59) and HE-4 (0.63) levels showed increased sensitivity (88.5%).
Conclusion: In ovarian and endometrial cancer, wherein early diagnosis is the most important factor for prognosis and survival, HE-4 is a new serum tumor marker that can be used with the aim of noninvasive diagnoses. For early diagnosis, the concomitant use of CA 125 and HE-4 is more effective and reliable than using either of them alone.
Amaç: CA 125 erken evre over kanserlerinin sadece %50’sinde yükselir. Endometrium kanserinde klinik pratikte rutin olarak kullanılan tümör marker yoktur. Human epididimis protein-4 (HE-4) over kanserlerinde yüksek sensitivite ve spesifiteye sahiptir. Endometrial kanserde CA 125 den daha sensitif olduğu ve erken evrede yükseldiği gösterilmiştir. Çalışmamızda CA 125 ve HE-4’ün over ve endometrium kanserinde tümör markerı olarak kullanılabilirliğini ve ikisinin birlikte kullanımın teşhis oranını ne şekilde etkileyeceğini araştırdık.
Gereç ve yöntem: Çalışmamıza histopatolojik olarak tanısı konulan 20 over kanseri, 26 endometrium kanseri hasta ile jinekolojik problemi olmayan 40 sağlıklı gönüllü dahil edilmiştir. MR görüntülemesiyle FIGO sistemine göre evreleri belirlendi. Hastalardan preoperatif olarak ve sağlıklı gönüllülerden periferik kan alınarak serum CA 125 ve HE-4 düzeyleri çalışıldı.
Bulgular: Over kanseri hastalarının serum CA 125 ve HE-4 düzeyleri sağlıklı kontrollerinkinden anlamlı yüksek bulundu (p<0,05). ROC (Receiver Operatör Characteristic) analizi uygulandığında over kanseri hastalarında CA 125 (0,83) ve HE-4 (0,84)’ün birlikte kullanımının sensitiviteyi arttırdığı (%95) görüldü. Endometrium kanseri hastaları ile sağlıklı kontrollerin CA 125 düzeyleri arasında anlamlı fark yok iken (p>0,05), HE-4 düzeyleri yüksek bulunmuştur (p<0,05). Endometrium kanseri hastaları ile sağlıklı kontroller arasında CA 125 (0,59) ve HE-4’ün (0,63) birlikte kullanımın sensitiviteyi (%88,5) arttırdığı gözlendi.
Sonuç: Erken teşhisin prognoz üzerindeki en önemli etken olduğu over ve endometrium kanserinde HE-4 non-invazif teşhis amacıyla kullanılabilecek tümör markerıdır. Erken teşhis amacıyla CA 125 ve HE-4’ün birlikte kullanımı herhangi birisinin tek başına kullanılmasına göre daha etkin ve güvenilirdir.
Keywords: Ovary; cancer; diagnosis; endometrium; tumor marker.
Conflict of interest statement
No conflict of interest was declared by the authors.
Figures
References
-
- Li J, Dowdy S, Tipton T, et al. HE4 as a biomarker for ovarian and endometrial cancer management. Expert Rev Mol Diagn. 2009;9:555–66. https://doi.org/10.1586/erm.09.39. - DOI - PMC - PubMed
-
- Zacharakis D, Thomakos N, Biliatis I, et al. Ultrasonographic markers and preoperative CA-125 to distinguish between borderline ovarian tumors and stage I ovarian cancer. Acta Obstet Gynecol Scand. 2013;92:285–92. https://doi.org/10.1111/aogs.12046. - DOI - PubMed
-
- Gadducci A, Tana R, Cosio S, Genazzani AR. The serum assay of tumour markers in the prognostic evaluation, treatment monitoring and follow-up of patients with cervical cancer: a review of the literature. Crit Rev Oncol Hematol. 2008;66:10–20. https://doi.org/10.1016/j.critrevonc.2007.09.002. - DOI - PubMed
-
- Farias-Eisner G, Su F, Robbins T, Kotlerman J, Reddy S, Farias-Eisner R. Validation of serum biomarkers for detection of early- and late-stage endometrial cancer. Am J Obstet Gynecol. 2010;202:73. https://doi.org/10.1016/j.ajog.2009.07.049. - DOI - PubMed
-
- Changes in definition of clinical staging for carcinoma of the cervix and ovary. Am J Obstet Gynecol. 1987;156:236. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
