Inner Hair Cell Loss Disrupts Hearing and Cochlear Function Leading to Sensory Deprivation and Enhanced Central Auditory Gain
- PMID: 28149271
- PMCID: PMC5241314
- DOI: 10.3389/fnins.2016.00621
Inner Hair Cell Loss Disrupts Hearing and Cochlear Function Leading to Sensory Deprivation and Enhanced Central Auditory Gain
Abstract
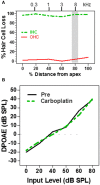
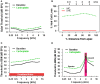
There are three times as many outer hair cells (OHC) as inner hair cells (IHC), yet IHC transmit virtually all acoustic information to the brain as they synapse with 90-95% of type I auditory nerve fibers. Here we review a comprehensive series of experiments aimed at determining how loss of the IHC/type I system affects hearing by selectively destroying these cells in chinchillas using the ototoxic anti-cancer agent carboplatin. Eliminating IHC/type I neurons has no effect on distortion product otoacoustic emission or the cochlear microphonic potential generated by OHC; however, it greatly reduces the summating potential produced by IHC and the compound action potential (CAP) generated by type I neurons. Remarkably, responses from remaining auditory nerve fibers maintain sharp tuning and low thresholds despite innervating regions of the cochlea with ~80% IHC loss. Moreover, chinchillas with large IHC lesions have surprisingly normal thresholds in quiet until IHC losses exceeded 80%, suggesting that only a few IHC are needed to detect sounds in quiet. However, behavioral thresholds in broadband noise are elevated significantly and tone-in-narrow band noise masking patterns exhibit greater remote masking. These results suggest the auditory system is able to compensate for considerable loss of IHC/type I neurons in quiet but not in difficult listening conditions. How does the auditory brain deal with the drastic loss of cochlear input? Recordings from the inferior colliculus found a relatively small decline in sound-evoked activity despite a large decrease in CAP amplitude after IHC lesion. Paradoxically, sound-evoked responses are generally larger than normal in the auditory cortex, indicative of increased central gain. This gain enhancement in the auditory cortex is associated with decreased GABA-mediated inhibition. These results suggest that when the neural output of the cochlea is reduced, the central auditory system compensates by turning up its gain so that weak signals once again become comfortably loud. While this gain enhancement is able to restore normal hearing under quiet conditions, it may not adequately compensate for peripheral dysfunction in more complex sound environments. In addition, excessive gain increases may convert recruitment into the debilitating condition known as hyperacusis.
Keywords: auditory cortex; auditory gain; carboplatin; central auditory system; hyperacusis; inner hair cells; tinnitus.
Figures






References
-
- Boettcher F., Gratton M. A., Bancroft B. R., Spongr V. (1992). Interaction of noise and other agents, in Noise Induced Hearing Loss, eds Dancer A., Henderson D., Salvi R., Hamernik R. P. (St. Louis, MO: Mosby Year Book; ), 175–187.
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

