Keeping It All Going-Complement Meets Metabolism
- PMID: 28149297
- PMCID: PMC5241319
- DOI: 10.3389/fimmu.2017.00001
Keeping It All Going-Complement Meets Metabolism
Abstract
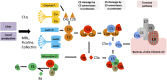
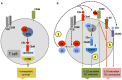
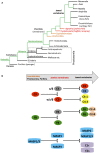
The complement system is an evolutionary old and crucial component of innate immunity, which is key to the detection and removal of invading pathogens. It was initially discovered as a liver-derived sentinel system circulating in serum, the lymph, and interstitial fluids that mediate the opsonization and lytic killing of bacteria, fungi, and viruses and the initiation of the general inflammatory responses. Although work performed specifically in the last five decades identified complement also as a critical instructor of adaptive immunity-indicating that complement's function is likely broader than initially anticipated-the dominant opinion among researchers and clinicians was that the key complement functions were in principle defined. However, there is now a growing realization that complement activity goes well beyond "classic" immune functions and that this system is also required for normal (neuronal) development and activity and general cell and tissue integrity and homeostasis. Furthermore, the recent discovery that complement activation is not confined to the extracellular space but occurs within cells led to the surprising understanding that complement is involved in the regulation of basic processes of the cell, particularly those of metabolic nature-mostly via novel crosstalks between complement and intracellular sensor, and effector, pathways that had been overlooked because of their spatial separation. These paradigm shifts in the field led to a renaissance in complement research and provide new platforms to now better understand the molecular pathways underlying the wide-reaching effects of complement functions in immunity and beyond. In this review, we will cover the current knowledge about complement's emerging relationship with the cellular metabolism machinery with a focus on the functional differences between serum-circulating versus intracellularly active complement during normal cell survival and induction of effector functions. We will also discuss how taking a closer look into the evolution of key complement components not only made the functional connection between complement and metabolism rather "predictable" but how it may also give clues for the discovery of additional roles for complement in basic cellular processes.
Keywords: T cells; complement; evolution; metabolic disease; metabolism.
Figures



References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

