The immunomodulatory anticancer agent, RRx-001, induces an interferon response through epigenetic induction of viral mimicry
- PMID: 28149332
- PMCID: PMC5270305
- DOI: 10.1186/s13148-017-0312-z
The immunomodulatory anticancer agent, RRx-001, induces an interferon response through epigenetic induction of viral mimicry
Abstract
Background: RRx-001, a dinitroazetidine derivative, is a novel anticancer agent currently in phase II clinical trials. It mediates immunomodulatory effects either directly through polarization of tumor associated macrophages or indirectly through vascular normalization and increased T-lymphocyte infiltration. With multiple additional mechanisms of action including upregulation of oxidative stress, depletion of GSH and NADPH, anti-angiogenesis and epigenetic modulation, RRx-001 is being studied as a radio- and chemo-sensitizer to resensitize tumors to prior therapy and to prime tumors to respond to radiation, chemotherapy and immunotherapy in combination therapy studies. Here, we identified another mechanism, viral mimicry, which refers to the "unsilencing" of epigenetically repressed viral genes present in the tumor that provokes an immune response and may contribute to the anticancer activity of RRx-001.
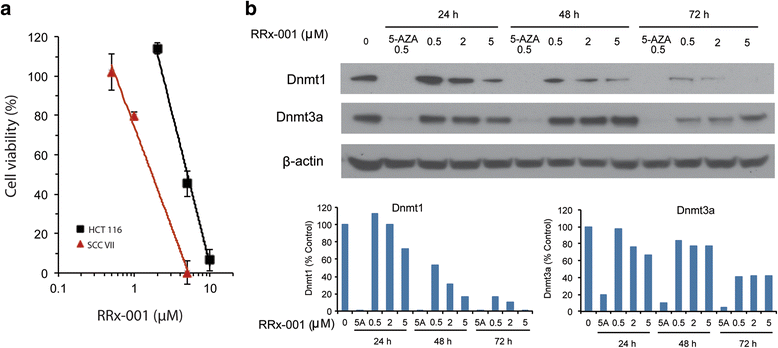
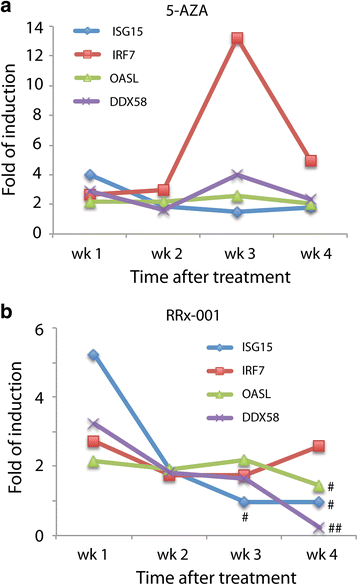
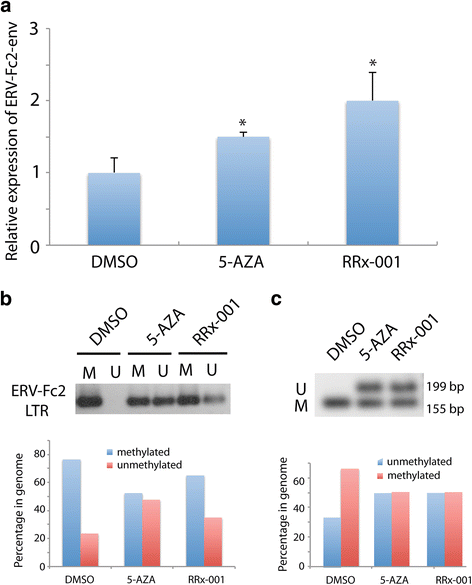
Results: RRx-001 inhibited the growth of colon cancer cells (HCT 116) and decreased levels of the DNA methyltransferases DNMT1 and DNMT3a in a time and dose-dependent manner. Treatment of HCT 116 cells with 0.5 μM RRx-001 for 24 h significantly increased transcripts of interferon (IFN)-responsive genes and this induction was sustained for up to 4 weeks after transient exposure to RRx-001. ELISA assays showed that RRx-001 increased secretion of type I and III IFNs by HCT 116 cells, and these IFNs were confirmed to be bioactive. Transcription of endogenous retrovirus ERV-Fc2 and LTRs from the ERV-L family (MLT2B4 and MLT1C49) was induced by RRx-001. The induction of ERV-Fc2-env was through demethylation of ERV-Fc2 LTR as determined by methylation-specific polymerase chain reaction and combined bisulfite restriction analysis. Immunofluorescence staining with J2 antibody confirmed induction of double-stranded RNA.
Conclusions: Transient exposure of HCT 116 cells to low-dose RRx-001 induced transcription of silenced retroviral genes present in the cancer cell DNA with subsequent synthesis of IFN in response to this "pseudo-pathogenic" stimulus, mimicking an antiviral defense. RRx-001-mediated IFN induction may have the potential to improve the efficacy of immunotherapies as well as radiotherapy, standard chemotherapies and molecularly targeted agents when used in combination. The striking safety profile of RRx-001 in comparison to other more toxic epigenetic and immunomodulatory agents such as azacitidine makes it a leading candidate for such clinical applications.
Keywords: Cancer; Epigenetics; Interferon response; Pseudo-infection; RRx-001; Viral mimicry.
Figures


References
-
- Herrera FG, Bourhis J, Coukos G. Radiotherapy combination opportunities leveraging immunity for the next oncology practice. CA: a cancer journal for clinicians. 2016. [Epub ahead of print] - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

