Scalable Synthesis of (-)-Thapsigargin
- PMID: 28149952
- PMCID: PMC5269647
- DOI: 10.1021/acscentsci.6b00313
Scalable Synthesis of (-)-Thapsigargin
Abstract
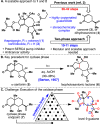
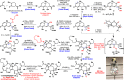
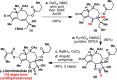
Total syntheses of the complex, highly oxygenated sesquiterpenes thapsigargin (1) and nortrilobolide (2) are presented. Access to analogues of these promising bioactive natural products has been limited to tedious isolation and semisynthetic efforts. Elegant prior total syntheses demonstrated the feasibility of creating these entitites in 36-42 step processes. The currently reported route proceeds in a scalable and more concise fashion by utilizing two-phase terpene synthesis logic. Salient features of the work include application of the classic photosantonin rearrangement and precisely choreographed installation of the multiple oxygenations present on the guaianolide skeleton.
Conflict of interest statement
The authors declare no competing financial interest.
Figures
References
-
- Rasmussen U.; Christensen S. B. Thapsigargine and thapsigargicine, two new histamine liberators from Thapsia garganica. Acta Pharm. Suec. 1978, 15, 133–140. - PubMed
-
- Tschirch A.; Stock E.. Die Harze; Verlag von Gebrüder Borntraeger: Berlin, 1936; Vol. 2, p 1540.
-
- Hecker E. Cocarcinogenic Principles from the Seed Oil of Croton tiglium and from Other Euphorbiaceae. Cancer Res. 1968, 28, 2338–2348. - PubMed
-
- Hecker E.; Bartsch H.; Bresch H.; Gschwendt M.; Härle B.; Kreibich G.; Kubinyi H.; Schairer H. U.; Szczepanski Ch. v.; Thielmann H. W. Structure and stereochemistry of the tetracyclic diterpene phorbol from croton tiglium L. Tetrahedron Lett. 1967, 8, 3165–3170. 10.1016/S0040-4039(01)89890-7. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

