Illuminating Phenylazopyridines To Photoswitch Metabotropic Glutamate Receptors: From the Flask to the Animals
- PMID: 28149957
- PMCID: PMC5269660
- DOI: 10.1021/acscentsci.6b00353
Illuminating Phenylazopyridines To Photoswitch Metabotropic Glutamate Receptors: From the Flask to the Animals
Abstract
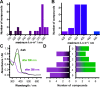
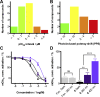
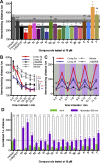
Phenylazopyridines are photoisomerizable compounds with high potential to control biological functions with light. We have obtained a series of phenylazopyridines with light dependent activity as negative allosteric modulators (NAM) of metabotropic glutamate receptor subtype 5 (mGlu5). Here we describe the factors needed to achieve an operational molecular photoisomerization and its effective translation into in vitro and in vivo receptor photoswitching, which includes zebrafish larva motility and the regulation of the antinociceptive effects in mice. The combination of light and some specific phenylazopyridine ligands displays atypical pharmacological profiles, including light-dependent receptor overactivation, which can be observed both in vitro and in vivo. Remarkably, the localized administration of light and a photoswitchable compound in the peripheral tissues of rodents or in the brain amygdalae results in an illumination-dependent analgesic effect. The results reveal a robust translation of the phenylazopyridine photoisomerization to a precise photoregulation of biological activity.
Conflict of interest statement
The authors declare no competing financial interest.
Figures





References
LinkOut - more resources
Full Text Sources
Other Literature Sources

