P-Selectin preserves immune tolerance in mice and is reduced in human cutaneous lupus
- PMID: 28150814
- PMCID: PMC5288776
- DOI: 10.1038/srep41841
P-Selectin preserves immune tolerance in mice and is reduced in human cutaneous lupus
Abstract
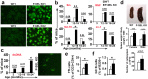
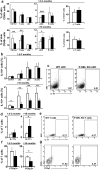
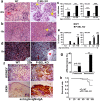
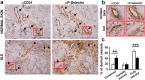
Mice deficient in P-Selectin presented altered immunity/tolerance balance. We have observed that the absence of P-Selectin promotes splenomegaly with reduced naïve T cell population, elevated activated/effector T cell subset, increased germinal center B and Tfh populations and high production of autoreactive antibodies. Moreover, 1.5-3-month-old P-selectin KO mice showed reduced IL-10-producing leukocytes in blood and a slightly reduced Treg population in the skin. With aging and, coinciding with disease severity, there is an increase in the IL17+ circulating and dermal T cell subpopulations and reduction of dermal Treg. As a consequence, P-Selectin deficient mice developed a progressive autoimmune syndrome showing skin alterations characteristic of lupus prone mice and elevated circulating autoantibodies, including anti-dsDNA. Similar to human SLE, disease pathogenesis was characterized by deposition of immune complexes in the dermoepidermal junction and renal glomeruli, and a complex pattern of autoantibodies. More important, skin biopsies of cutaneous lupus erythematosus patients did not show increased expression of P-Selectin, as described for other inflammatory diseases, and the number of vessels expressing P-Selectin was reduced.
Conflict of interest statement
The authors declare no competing financial interests.
Figures


References
-
- Vestweber D. & Blanks J. Mechanisms that regulate the function of the selectins and their ligands. Physiol Rev 79, 181–213 (1999). - PubMed
-
- Wagner J. G. & Roth R. A. Neutrophil Migration Mechanisms, with an Emphasis on the Pulmonary Vasculature. Pharmacol Rev 52, 349–374 (2000). - PubMed
-
- Ley K. The role of selectins in inflammation and disease. Trends Mol Med 9, 263–268 (2003). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

