Bone marrow microenvironment-derived signals induce Mcl-1 dependence in multiple myeloma
- PMID: 28151428
- PMCID: PMC5383873
- DOI: 10.1182/blood-2016-10-745059
Bone marrow microenvironment-derived signals induce Mcl-1 dependence in multiple myeloma
Abstract
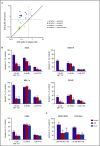
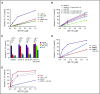
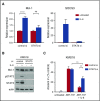
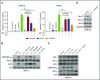
Multiple myeloma is highly dependent on the bone marrow microenvironment until progressing to very advanced extramedullary stages of the disease such as plasma cell leukemia. Stromal cells in the bone marrow secrete a variety of cytokines that promote plasma cell survival by regulating antiapoptotic members of the Bcl-2 family including Mcl-1, Bcl-xL, and Bcl-2. Although the antiapoptotic protein on which a cell depends is typically consistent among normal cells of a particular phenotype, Bcl-2 family dependence is highly heterogeneous in multiple myeloma. Although normal plasma cells and most multiple myeloma cells require Mcl-1 for survival, a subset of myeloma is codependent on Bcl-2 and/or Bcl-xL We investigated the role of the bone marrow microenvironment in determining Bcl-2 family dependence in multiple myeloma. We used the Bcl-2/Bcl-xL inhibitor ABT-737 to study the factors regulating whether myeloma is Mcl-1 dependent, and thus resistant to ABT-737-induced apoptosis, or Bcl-2/Bcl-xL codependent, and thus sensitive to ABT-737. We demonstrate that bone marrow stroma is capable of inducing Mcl-1 dependence through the production of the plasma cell survival cytokine interleukin-6 (IL-6). IL-6 upregulates Mcl-1 transcription in a STAT3-dependent manner, although this occurred in a minority of the cells tested. In all cells, IL-6 treatment results in posttranslational modification of the proapoptotic protein Bim. Phosphorylation of Bim shifts its binding from Bcl-2 and Bcl-xL to Mcl-1, an effect reversed by MEK inhibition. Blocking IL-6 or downstream signaling restored Bcl-2/Bcl-xL dependence and may therefore represent a clinically useful strategy to enhance the activity of Bcl-2 inhibitors.
© 2017 by The American Society of Hematology.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

