Clinical and molecular consequences of disease-associated de novo mutations in SATB2
- PMID: 28151491
- PMCID: PMC5548934
- DOI: 10.1038/gim.2016.211
Clinical and molecular consequences of disease-associated de novo mutations in SATB2
Abstract
Purpose: To characterize features associated with de novo mutations affecting SATB2 function in individuals ascertained on the basis of intellectual disability.
Methods: Twenty previously unreported individuals with 19 different SATB2 mutations (11 loss-of-function and 8 missense variants) were studied. Fibroblasts were used to measure mutant protein production. Subcellular localization and mobility of wild-type and mutant SATB2 were assessed using fluorescently tagged protein.
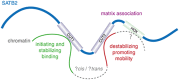
Results: Recurrent clinical features included neurodevelopmental impairment (19/19), absent/near absent speech (16/19), normal somatic growth (17/19), cleft palate (9/19), drooling (12/19), and dental anomalies (8/19). Six of eight missense variants clustered in the first CUT domain. Sibling recurrence due to gonadal mosaicism was seen in one family. A nonsense mutation in the last exon resulted in production of a truncated protein retaining all three DNA-binding domains. SATB2 nuclear mobility was mutation-dependent; p.Arg389Cys in CUT1 increased mobility and both p.Gly515Ser in CUT2 and p.Gln566Lys between CUT2 and HOX reduced mobility. The clinical features in individuals with missense variants were indistinguishable from those with loss of function.
Conclusion: SATB2 haploinsufficiency is a common cause of syndromic intellectual disability. When mutant SATB2 protein is produced, the protein appears functionally inactive with a disrupted pattern of chromatin or matrix association.Genet Med advance online publication 02 February 2017.
Figures



References
-
- FitzPatrick DR, Carr IM, McLaren L, et al. Identification of SATB2 as the cleft palate gene on 2q32-q33. Hum Mol Genet 2003;12:2491–2501. - PubMed
-
- Dobreva G, Chahrour M, Dautzenberg M, et al. SATB2 is a multifunctional determinant of craniofacial patterning and osteoblast differentiation. Cell 2006;125:971–986. - PubMed
-
- Alcamo EA, Chirivella L, Dautzenberg M, et al. Satb2 regulates callosal projection neuron identity in the developing cerebral cortex. Neuron 2008;57:364–377. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

