Integration of Novel Agents into the Care of Patients with Multiple Myeloma
- PMID: 28151712
- PMCID: PMC5705234
- DOI: 10.1158/1078-0432.CCR-16-0861
Integration of Novel Agents into the Care of Patients with Multiple Myeloma
Erratum in
-
Correction: Integration of Novel Agents into the Care of Patients with Multiple Myeloma.Clin Cancer Res. 2017 May 15;23(10):2605. doi: 10.1158/1078-0432.CCR-17-0064. Clin Cancer Res. 2017. PMID: 28507271 No abstract available.
Abstract
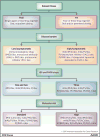
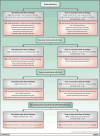
The pace of therapeutic drug development in multiple myeloma has reached unprecedented levels, with five regulatory approvals for relapsed and/or refractory disease of either new drugs or new drug regimens in 2015, one already in 2016, and still others anticipated. This has provided a wide array of options to be considered by patients and their health care providers in the event of relapse after or progression on front-line therapy. Most of these agents are currently being evaluated in earlier patient populations, including as parts of induction, consolidation, and maintenance therapy approaches, where their benefits may be even greater. Moreover, additional randomized studies have been completed with our previous stable of novel agents that inform their use in these settings as well. In the current contribution to this CCR Focus on multiple myeloma, we will present an overview of some of the key recent data that have supported the addition of these new therapeutics to our armamentarium against multiple myeloma. Also, we will provide some guidelines about possible best practices in applying these regimens and attempt to extrapolate how they will be used as parts of our future standards of care. Clin Cancer Res; 22(22); 5443-52. ©2016 AACR SEE ALL ARTICLES IN THIS CCR FOCUS SECTION, "MULTIPLE MYELOMA MULTIPLYING THERAPIES".
©2016 American Association for Cancer Research.
Conflict of interest statement
No other potential conflicts of interest were disclosed.
Figures
References
-
- Surveillance, Epidemiology, and End Results Program [database on the Internet] Bethesda (MD): National Cancer Institute; 2016. [[cited 2016 Sep 20]]. Available from: http://seer.cancer.gov.
-
- GLOBOCAN 2012: Estimated Cancer incidence, Mortality and Prevalence Worldwide in 2012 [database on the Internet] Lyon (France): International Agency for Research on Cancer; 2016. [[cited 2016 Sep 20]]. Available from: http://globocan.iarc.fr/Default.aspx.
-
- Smith BD, Smith GL, Hurria A, Hortobagyi GN, Buchholz TA. Future of cancer incidence in the United States: burdens upon an aging, changing nation. Journal of clinical oncology. 2009;27:2758–65. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

