Does Training and Support of General Practitioners in Intensive Treatment of People with Screen-Detected Diabetes Improve Medication, Morbidity and Mortality in People with Clinically-Diagnosed Diabetes? Investigation of a Spill-Over Effect in a Cluster RCT
- PMID: 28151941
- PMCID: PMC5289474
- DOI: 10.1371/journal.pone.0170697
Does Training and Support of General Practitioners in Intensive Treatment of People with Screen-Detected Diabetes Improve Medication, Morbidity and Mortality in People with Clinically-Diagnosed Diabetes? Investigation of a Spill-Over Effect in a Cluster RCT
Abstract
Introduction: Very few studies have examined the potential spill-over effect of a trial intervention in general practice. We investigated whether training and support of general practitioners in the intensive treatment of people with screen-detected diabetes improved rates of redeemed medication, morbidity and mortality in people with clinically-diagnosed diabetes.
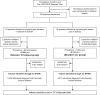
Methods: This is a secondary, post-hoc, register-based analysis linked to a cluster randomised trial. In the ADDITION-Denmark trial, 175 general practices were cluster randomised (i) to routine care, or (ii) to receive training and support in intensive multifactorial treatment of individuals with screen-detected diabetes (2001 to 2009). Using national registers we identified all individuals who were diagnosed with clinically incident diabetes in the same practices over the same time period. (Patients participating in the ADDITION trial were excluded). We compared rates of redeemed medication, a cardiovascular composite endpoint, and all-cause mortality between the routine care and intensive treatment groups.
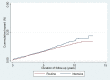
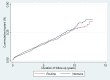
Results: In total, 4,107 individuals were diagnosed with clinically incident diabetes in ADDITION-Denmark practices between 2001 and 2009 (2,051 in the routine care group and 2,056 in the intensive treatment group). There were large and significant increases in the proportion of patients redeeming cardio-protective medication in both treatment groups during follow-up. After a median of seven years of follow-up, there was no difference in the incidence of a composite cardiovascular endpoint (HR 1.15, 95% CI 0.95 to 1.38) or all-cause mortality between the two groups (HR 1.08, 95% CI 0.94 to 1.23).
Discussion: There was no evidence of a spill-over effect from an intervention promoting intensive treatment of people with screen-detected diabetes to those with clinically-diagnosed diabetes. Overall, the proportion of patients redeeming cardio-protective medication during follow-up was similar in both groups.
Trial registration: ClinicalTrials.gov NCT00237549.
Conflict of interest statement
MC is supported by the Novo Nordisk Foundation. DRW and RKS are supported by the Danish Diabetes Academy, which is funded by the Novo Nordisk Foundation. DRW reports receiving lecture fees from Novo Nordisk A/S and Steno Diabetes Center. DRW and TL hold shares in Novo Nordisk A/S. TL reports receiving a fee for attending an international board meeting for Astra Zeneca on early detection and treatment of diabetes in 2015. AS reports receiving lecture fees for providing continuing medical education to GPs. SJG’s research programme is supported by MRC Epidemiology Unit core funding (MC_UU_12015/4). SJG is an NIHR Senior Investigator and member of the NIHR School for Primary Care Research. SJG receives an honorarium and reimbursement of travel expenses from Eli Lilly associated with membership of an independent data monitoring committee for a randomised trial of a medication to lower glucose. SJG received an honorarium from Janssen for speaking at an educational meeting in 2015. This does not alter our adherence to PLOS ONE policies on sharing data and materials.
Figures
References
-
- 2016) Standards of Medical Care in Diabetes-2016. Diabetes Care 39 Suppl 1: S1–S2. 39/Supplement_1/S1 [pii];. - PubMed
-
- Grimshaw JM, Thomas RE, MacLennan G, Fraser C, Ramsay CR, Vale L, Whitty P, Eccles MP, Matowe L, Shirran L, Wensing M, Dijkstra R, Donaldson C (2004) Effectiveness and efficiency of guideline dissemination and implementation strategies. Health Technol Assess 8: iii–72. 94-08-29 [pii]. - PubMed
-
- Lauritzen T, Griffin S, Borch-Johnsen K, Wareham NJ, Wolffenbuttel BH, Rutten G (2000) The ADDITION study: proposed trial of the cost-effectiveness of an intensive multifactorial intervention on morbidity and mortality among people with Type 2 diabetes detected by screening. Int J Obes Relat Metab Disord 24 Suppl 3: S6–11. - PubMed
Publication types
MeSH terms
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

