Identification of small molecules that disrupt vacuolar function in the pathogen Candida albicans
- PMID: 28151949
- PMCID: PMC5289544
- DOI: 10.1371/journal.pone.0171145
Identification of small molecules that disrupt vacuolar function in the pathogen Candida albicans
Abstract
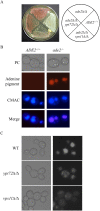
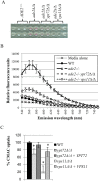
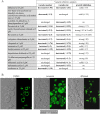
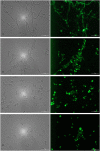
The fungal vacuole is a large acidified organelle that performs a variety of cellular functions. At least a sub-set of these functions are crucial for pathogenic species of fungi, such as Candida albicans, to survive within and invade mammalian tissue as mutants with severe defects in vacuolar biogenesis are avirulent. We therefore sought to identify chemical probes that disrupt the normal function and/or integrity of the fungal vacuole to provide tools for the functional analysis of this organelle as well as potential experimental therapeutics. A convenient indicator of vacuolar integrity based upon the intracellular accumulation of an endogenously produced pigment was adapted to identify Vacuole Disrupting chemical Agents (VDAs). Several chemical libraries were screened and a set of 29 compounds demonstrated to reproducibly cause loss of pigmentation, including 9 azole antifungals, a statin and 3 NSAIDs. Quantitative analysis of vacuolar morphology revealed that (excluding the azoles) a sub-set of 14 VDAs significantly alter vacuolar number, size and/or shape. Many C. albicans mutants with impaired vacuolar function are deficient in the formation of hyphal elements, a process essential for its pathogenicity. Accordingly, all 14 VDAs negatively impact C. albicans hyphal morphogenesis. Fungal selectivity was observed for approximately half of the VDA compounds identified, since they did not alter the morphology of the equivalent mammalian organelle, the lysosome. Collectively, these compounds comprise of a new collection of chemical probes that directly or indirectly perturb normal vacuolar function in C. albicans.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures

Similar articles
-
ERG2 and ERG24 Are Required for Normal Vacuolar Physiology as Well as Candida albicans Pathogenicity in a Murine Model of Disseminated but Not Vaginal Candidiasis.Eukaryot Cell. 2015 Oct;14(10):1006-16. doi: 10.1128/EC.00116-15. Epub 2015 Jul 31. Eukaryot Cell. 2015. PMID: 26231054 Free PMC article.
-
The Vacuolar Ca2+ ATPase Pump Pmc1p Is Required for Candida albicans Pathogenesis.mSphere. 2019 Feb 6;4(1):e00715-18. doi: 10.1128/mSphere.00715-18. mSphere. 2019. PMID: 30728284 Free PMC article.
-
Subunits of the vacuolar H+-ATPase complex, Vma4 and Vma10, are essential for virulence and represent potential drug targets in Candida albicans.Fungal Biol. 2019 Oct;123(10):709-722. doi: 10.1016/j.funbio.2019.06.002. Epub 2019 Jun 12. Fungal Biol. 2019. PMID: 31542189
-
Sterols in Candida albicans mutants resistant to polyene or azole antifungals, and of a double mutant C. albicans 6.4.Crit Rev Microbiol. 1987;15(1):111-5. doi: 10.3109/10408418709104454. Crit Rev Microbiol. 1987. PMID: 3319418 Review.
-
Resistance in human pathogenic yeasts and filamentous fungi: prevalence, underlying molecular mechanisms and link to the use of antifungals in humans and the environment.Dan Med J. 2016 Oct;63(10):B5288. Dan Med J. 2016. PMID: 27697142 Review.
Cited by
-
In Vitro Incorporation of Helicobacter pylori into Candida albicans Caused by Acidic pH Stress.Pathogens. 2020 Jun 19;9(6):489. doi: 10.3390/pathogens9060489. Pathogens. 2020. PMID: 32575493 Free PMC article.
-
A mechanism of lysosomal calcium entry.Sci Adv. 2024 Feb 16;10(7):eadk2317. doi: 10.1126/sciadv.adk2317. Epub 2024 Feb 14. Sci Adv. 2024. PMID: 38354239 Free PMC article.
-
Drug Repurposing in Medical Mycology: Identification of Compounds as Potential Antifungals to Overcome the Emergence of Multidrug-Resistant Fungi.Pharmaceuticals (Basel). 2021 May 20;14(5):488. doi: 10.3390/ph14050488. Pharmaceuticals (Basel). 2021. PMID: 34065420 Free PMC article. Review.
-
Weapons against Themselves: Identification and Use of Quorum Sensing Volatile Molecules to Control Plant Pathogenic Fungi Growth.Microorganisms. 2022 Dec 13;10(12):2459. doi: 10.3390/microorganisms10122459. Microorganisms. 2022. PMID: 36557712 Free PMC article.
-
Can nonsteroidal anti-inflammatory drugs (NSAIDs) be repurposed for fungal infection?Naunyn Schmiedebergs Arch Pharmacol. 2024 Jan;397(1):59-75. doi: 10.1007/s00210-023-02651-x. Epub 2023 Aug 17. Naunyn Schmiedebergs Arch Pharmacol. 2024. PMID: 37589736 Review.
References
-
- Maertens J, Vrebos M, Boogaerts M. 2001. Assessing risk factors for systemic fungal infections. Eur J Cancer Care (Engl) 10:56–62. - PubMed
-
- Goodwin SD, Cleary JD, Walawander CA, Taylor JW, Grasela TH Jr. 1995. Pretreatment regimens for adverse events related to infusion of amphotericin B. Clin Infect Dis 20:755–761. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

