Comparison of corneal epitheliotrophic capacities among human platelet lysates and other blood derivatives
- PMID: 28152010
- PMCID: PMC5289502
- DOI: 10.1371/journal.pone.0171008
Comparison of corneal epitheliotrophic capacities among human platelet lysates and other blood derivatives
Abstract
Purpose: To evaluate the corneal epitheliotropic abilities of two commercialized human platelet lysates (HPLs) and to compare the results with other blood derivatives, including human peripheral serum (HPS) and bovine fetal serum (FBS).
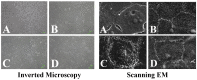
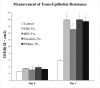
Methods: In vitro, human corneal epithelial cells were incubated in various concentrations (0%, 3%, 5% and 10%) of blood derivatives. Two commercialized HPLs, including UltraGRO TM (Helios, Atlanta, GA) and PLTMax (Mill Creek, Rochester, MI), were tested and compared with HPS and FBS. Scratch-induced directional wounding assay was performed to evaluate cellular migration. MTS assay was used to evaluate cellular proliferation. Cellular differentiation was examined by scanning electron microscopy, inverted microscopy and transepithelial electrical resistance. Sprague-Dawley rats were used to evaluate the effects of the blood derivatives on corneal epithelial wound healing in vivo. Different blood derivatives were applied topically every 2 hours for 2 days after corneal epithelial debridement. The concentrations of epidermal growth factor (EGF), transforming growth factor -β1 (TGF-β1), fibronectin, platelet-derived growth factor-AB (PDGF-AB), PDGF-BB, and hyaluronic acid in different blood derivatives were evaluated by enzyme-linked immunosorbent assay (ELISA).
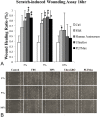
Results: In vitro experiments demonstrated statistically comparable epitheliotropic characteristics in cellular proliferation, migration, and differentiation for the two commercialized HPLs compared to FBS and HPS. Cells cultured without any serum were used as control group. The epitheliotropic capacities were statistically higher in the two commercialized HPLs compared to the control group (p<0.05). Among the different concentrations of blood derivatives, the preparations with 3% yielded better outcomes compared to 5% and 10%. In rats, HPLs also caused improved but not statistically significant wound healing compared to HPS. All the blood derivatives had better wound healing ratios than the control group (p<0.05). In the quantification of epitheliotropic factors, UltraGRO and PLTMax had significantly higher levels of EGF, TGF- β1, fibronectin than human peripheral serum (p<0.05).
Conclusions: Both commercialized HPLs showed comparable corneal epitheliotropic abilities and wound healing rates compared to HPS and FBS in the in vivo and in vitro studies. Our results suggest that HPLs may have the potential to replace HPS in the treatment of corneal epithelial problems.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures


Similar articles
-
The corneal epitheliotrophic abilities of lyophilized powder form human platelet lysates.PLoS One. 2018 Mar 16;13(3):e0194345. doi: 10.1371/journal.pone.0194345. eCollection 2018. PLoS One. 2018. PMID: 29547658 Free PMC article.
-
Comparison of epitheliotrophic factors in autologous serum eyedrops from sera of chronic renal failure patients vs. normal controls.Graefes Arch Clin Exp Ophthalmol. 2015 Oct;253(10):1705-12. doi: 10.1007/s00417-015-3056-5. Epub 2015 May 31. Graefes Arch Clin Exp Ophthalmol. 2015. PMID: 26026935
-
The effect of human platelet lysate on corneal nerve regeneration.Br J Ophthalmol. 2021 Jun;105(6):884-890. doi: 10.1136/bjophthalmol-2019-314408. Epub 2019 Nov 20. Br J Ophthalmol. 2021. PMID: 31748333
-
Corneal epitheliotrophic capacity of three different blood-derived preparations.Invest Ophthalmol Vis Sci. 2006 Jun;47(6):2438-44. doi: 10.1167/iovs.05-0876. Invest Ophthalmol Vis Sci. 2006. PMID: 16723454
-
Effects of growth factors (EGF, PDGF-BB and TGF-beta 1) on cultured equine epithelial cells and keratocytes: implications for wound healing.Vet Ophthalmol. 2003 Sep;6(3):211-7. doi: 10.1046/j.1463-5224.2003.00296.x. Vet Ophthalmol. 2003. PMID: 12950652
Cited by
-
Treatment of Non-Infectious Corneal Injury: Review of Diagnostic Agents, Therapeutic Medications, and Future Targets.Drugs. 2022 Feb;82(2):145-167. doi: 10.1007/s40265-021-01660-5. Epub 2022 Jan 13. Drugs. 2022. PMID: 35025078 Free PMC article. Review.
-
Platelet lysate promotes re-epithelialization of persistent epithelial defects: a pilot study.Int Ophthalmol. 2019 Jul;39(7):1483-1490. doi: 10.1007/s10792-018-0968-1. Epub 2018 Jul 5. Int Ophthalmol. 2019. PMID: 29978342
-
Epithelial Cell Migration and Proliferation Patterns During Initial Wound Closure in Normal Mice and an Experimental Model of Limbal Stem Cell Deficiency.Invest Ophthalmol Vis Sci. 2020 Aug 3;61(10):27. doi: 10.1167/iovs.61.10.27. Invest Ophthalmol Vis Sci. 2020. PMID: 32790859 Free PMC article.
-
Therapy for corneal diseases, layer by layer.Taiwan J Ophthalmol. 2017 Oct-Dec;7(4):177-178. doi: 10.4103/2211-5056.219939. Taiwan J Ophthalmol. 2017. PMID: 29296548 Free PMC article. No abstract available.
-
Differences between the Proliferative Effects of Human Platelet Lysate and Fetal Bovine Serum on Human Adipose-Derived Stem Cells.Cells. 2019 Oct 8;8(10):1218. doi: 10.3390/cells8101218. Cells. 2019. PMID: 31597348 Free PMC article.
References
-
- Noda-Tsuruya T, Asano-Kato N, Toda I, Tsubota K. Autologous serum eye drops for dry eye after LASIK. Journal of refractive surgery. 2006;22(1):61–6. - PubMed
-
- Hartwig D, Herminghaus P, Wedel T, Liu L, Schlenke P, Dibbelt L, et al. Topical treatment of ocular surface defects: comparison of the epitheliotrophic capacity of fresh frozen plasma and serum on corneal epithelial cells in an in vitro cell culture model. Transfusion medicine. 2005;15(2):107–13. 10.1111/j.0958-7578.2005.00559.x - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

