Apigenin: Selective CK2 inhibitor increases Ikaros expression and improves T cell homeostasis and function in murine pancreatic cancer
- PMID: 28152014
- PMCID: PMC5289423
- DOI: 10.1371/journal.pone.0170197
Apigenin: Selective CK2 inhibitor increases Ikaros expression and improves T cell homeostasis and function in murine pancreatic cancer
Abstract
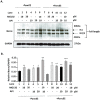
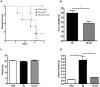
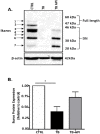
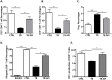
Pancreatic cancer (PC) evades immune destruction by favoring the development of regulatory T cells (Tregs) that inhibit effector T cells. The transcription factor Ikaros is critical for lymphocyte development, especially T cells. We have previously shown that downregulation of Ikaros occurs as a result of its protein degradation by the ubiquitin-proteasome system in our Panc02 tumor-bearing (TB) mouse model. Mechanistically, we observed a deregulation in the balance between Casein Kinase II (CK2) and protein phosphatase 1 (PP1), which suggested that increased CK2 activity is responsible for regulating Ikaros' stability in our model. We also showed that this loss of Ikaros expression is associated with a significant decrease in CD4+ and CD8+ T cell percentages but increased CD4+CD25+ Tregs in TB mice. In this study, we evaluated the effects of the dietary flavonoid apigenin (API), on Ikaros expression and T cell immune responses. Treatment of splenocytes from naïve mice with (API) stabilized Ikaros expression and prevented Ikaros downregulation in the presence of murine Panc02 cells in vitro, similar to the proteasome inhibitor MG132. In vivo treatment of TB mice with apigenin (TB-API) improved survival, reduced tumor weights and prevented splenomegaly. API treatment also restored protein expression of some Ikaros isoforms, which may be attributed to its moderate inhibition of CK2 activity from splenocytes of TB-API mice. This partial restoration of Ikaros expression was accompanied by a significant increase in CD4+ and CD8+ T cell percentages and a reduction in Treg percentages in TB-API mice. In addition, CD8+ T cells from TB-API mice produced more IFN-γ and their splenocytes were better able to prime allogeneic CD8+ T cell responses compared to TB mice. These results provide further evidence that Ikaros is regulated by CK2 in our pancreatic cancer model. More importantly, our findings suggest that API may be a possible therapeutic agent for stabilizing Ikaros expression and function to maintain T cell homeostasis in murine PC.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures

Similar articles
-
Murine pancreatic adenocarcinoma reduces Ikaros expression and disrupts T cell homeostasis.PLoS One. 2015 Jan 28;10(1):e0115546. doi: 10.1371/journal.pone.0115546. eCollection 2015. PLoS One. 2015. PMID: 25629611 Free PMC article.
-
Ikaros, CK2 kinase, and the road to leukemia.Mol Cell Biochem. 2011 Oct;356(1-2):201-7. doi: 10.1007/s11010-011-0964-5. Epub 2011 Jul 13. Mol Cell Biochem. 2011. PMID: 21750978 Free PMC article. Review.
-
Protein phosphatase 1 (PP1) and Casein Kinase II (CK2) regulate Ikaros-mediated repression of TdT in thymocytes and T-cell leukemia.Pediatr Blood Cancer. 2014 Dec;61(12):2230-5. doi: 10.1002/pbc.25221. Epub 2014 Sep 11. Pediatr Blood Cancer. 2014. PMID: 25214003 Free PMC article.
-
Regulation of cellular proliferation in acute lymphoblastic leukemia by Casein Kinase II (CK2) and Ikaros.Adv Biol Regul. 2017 Jan;63:71-80. doi: 10.1016/j.jbior.2016.09.003. Epub 2016 Sep 18. Adv Biol Regul. 2017. PMID: 27666503 Free PMC article. Review.
-
Murine pancreatic adenocarcinoma dampens SHIP-1 expression and alters MDSC homeostasis and function.PLoS One. 2011;6(11):e27729. doi: 10.1371/journal.pone.0027729. Epub 2011 Nov 22. PLoS One. 2011. PMID: 22132131 Free PMC article.
Cited by
-
Immunomodulatory potential of natural products from herbal medicines as immune checkpoints inhibitors: Helping to fight against cancer via multiple targets.Med Res Rev. 2022 May;42(3):1246-1279. doi: 10.1002/med.21876. Epub 2022 Jan 14. Med Res Rev. 2022. PMID: 35028953 Free PMC article. Review.
-
Unexpected Binding Mode of a Potent Indeno[1,2-b]indole-Type Inhibitor of Protein Kinase CK2 Revealed by Complex Structures with the Catalytic Subunit CK2α and Its Paralog CK2α'.Pharmaceuticals (Basel). 2017 Dec 13;10(4):98. doi: 10.3390/ph10040098. Pharmaceuticals (Basel). 2017. PMID: 29236079 Free PMC article.
-
MicroRNA-155 and its exosomal form: Small pieces in the gastrointestinal cancers puzzle.Cell Biol Toxicol. 2024 Sep 16;40(1):77. doi: 10.1007/s10565-024-09920-2. Cell Biol Toxicol. 2024. PMID: 39283408 Free PMC article. Review.
-
Modulatory Role of Phytochemicals/Natural Products in Cancer Immunotherapy.Curr Med Chem. 2024;31(32):5165-5177. doi: 10.2174/0109298673274796240116105555. Curr Med Chem. 2024. PMID: 38549529 Review.
-
Apigenin in cancer therapy: anti-cancer effects and mechanisms of action.Cell Biosci. 2017 Oct 5;7:50. doi: 10.1186/s13578-017-0179-x. eCollection 2017. Cell Biosci. 2017. PMID: 29034071 Free PMC article. Review.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

