Nik-related kinase regulates trophoblast proliferation and placental development by modulating AKT phosphorylation
- PMID: 28152035
- PMCID: PMC5289614
- DOI: 10.1371/journal.pone.0171503
Nik-related kinase regulates trophoblast proliferation and placental development by modulating AKT phosphorylation
Abstract
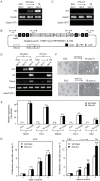
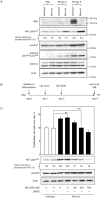
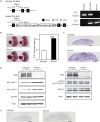
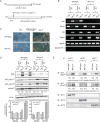
Nik-related kinase (Nrk) is a Ser/Thr kinase and was initially discovered as a molecule that was predominantly detected in skeletal muscles during development. A recent study using Nrk-null mice suggested the importance of Nrk in proper placental development; however, the molecular mechanism remains unknown. In this study, we demonstrated that differentiated trophoblasts from murine embryonic stem cells (ESCs) endogenously expressed Nrk and that Nrk disruption led to the enhanced proliferation of differentiated trophoblasts. This phenomenon may reflect the overproliferation of trophoblasts that has been reported in enlarged placentas of Nrk-null mice. Furthermore, we demonstrated that AKT phosphorylation at Ser473 was upregulated in Nrk-null trophoblasts and that inhibition of AKT phosphorylation cancelled the enhanced proliferation observed in differentiated Nrk-null trophoblasts. These results indicated that the upregulation of AKT phosphorylation was the possible cause of enhanced proliferation observed in Nrk-null trophoblasts. The upregulation of AKT phosphorylation was also confirmed in enlarged Nrk-null placentas in vivo, suggesting that proper regulation of AKT by Nrk was important for normal placental development. In addition, our detailed analysis on phosphorylation status of AKT isoforms in newly established trophoblast stem cells (TSCs) revealed that different levels of upregulation of AKT phosphorylation were occurred in Nrk-null TSCs depending on AKT isoforms. These results further support the importance of Nrk in proper development of trophoblast lineage cells and indicate the possible application of TSCs for the analysis of differently regulated activation mechanisms of AKT isoforms.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
Similar articles
-
A possible function of Nik-related kinase in the labyrinth layer of delayed delivery mouse placentas.J Reprod Dev. 2023 Feb 8;69(1):32-40. doi: 10.1262/jrd.2022-120. Epub 2022 Dec 24. J Reprod Dev. 2023. PMID: 36567126 Free PMC article.
-
Placental Mammals Acquired Functional Sequences in NRK for Regulating the CK2-PTEN-AKT Pathway and Placental Cell Proliferation.Mol Biol Evol. 2022 Feb 3;39(2):msab371. doi: 10.1093/molbev/msab371. Mol Biol Evol. 2022. PMID: 34999820 Free PMC article.
-
FBXL12-Mediated Degradation of ALDH3 is Essential for Trophoblast Differentiation During Placental Development.Stem Cells. 2015 Nov;33(11):3327-40. doi: 10.1002/stem.2088. Epub 2015 Jul 14. Stem Cells. 2015. PMID: 26124079
-
Trophoblast stem cells: models for investigating trophectoderm differentiation and placental development.Endocr Rev. 2009 May;30(3):228-40. doi: 10.1210/er.2009-0001. Epub 2009 Mar 18. Endocr Rev. 2009. PMID: 19299251 Free PMC article. Review.
-
Transcriptional regulators of the trophoblast lineage in mammals with hemochorial placentation.Reproduction. 2014 Dec;148(6):R121-36. doi: 10.1530/REP-14-0072. Epub 2014 Sep 4. Reproduction. 2014. PMID: 25190503 Free PMC article. Review.
Cited by
-
Rare variant associations with birth weight identify genes involved in adipose tissue regulation, placental function and insulin-like growth factor signalling.medRxiv [Preprint]. 2024 Apr 3:2024.04.03.24305248. doi: 10.1101/2024.04.03.24305248. medRxiv. 2024. Update in: Nat Commun. 2025 Jan 14;16(1):648. doi: 10.1038/s41467-024-55761-2. PMID: 38633783 Free PMC article. Updated. Preprint.
-
Expression of Nik-related kinase in smooth muscle cells attenuates vascular inflammation and intimal hyperplasia.Aging (Albany NY). 2020 Apr 24;12(8):7511-7533. doi: 10.18632/aging.103104. Epub 2020 Apr 24. Aging (Albany NY). 2020. PMID: 32330120 Free PMC article.
-
miR-338-5p Targets Epidermal Growth Factor-Containing Fibulin-Like Extracellular Matrix Protein 1 to Inhibit the Growth and Invasion of Trophoblast Cells in Selective Intrauterine Growth Restriction.Reprod Sci. 2020 Jun;27(6):1357-1364. doi: 10.1007/s43032-020-00160-3. Epub 2020 Feb 13. Reprod Sci. 2020. PMID: 32056133 Free PMC article.
-
A possible function of Nik-related kinase in the labyrinth layer of delayed delivery mouse placentas.J Reprod Dev. 2023 Feb 8;69(1):32-40. doi: 10.1262/jrd.2022-120. Epub 2022 Dec 24. J Reprod Dev. 2023. PMID: 36567126 Free PMC article.
-
Identification and functional activity of Nik related kinase (NRK) in benign hyperplastic prostate.J Transl Med. 2024 Mar 9;22(1):255. doi: 10.1186/s12967-024-05048-3. J Transl Med. 2024. PMID: 38459501 Free PMC article.
References
-
- Cross JC. How to make a placenta: mechanisms of trophoblast cell differentiation in mice—a review. Placenta. 2005; 26 Suppl A: S3–9. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

