Effectiveness and tolerance of single tablet versus once daily multiple tablet regimens as first-line antiretroviral therapy - Results from a large french multicenter cohort study
- PMID: 28152047
- PMCID: PMC5289500
- DOI: 10.1371/journal.pone.0170661
Effectiveness and tolerance of single tablet versus once daily multiple tablet regimens as first-line antiretroviral therapy - Results from a large french multicenter cohort study
Abstract
Objectives: Pill burden during antiretroviral treatment (ART) is associated with worse adherence and impaired virological suppression. We compared the effectiveness, tolerance, and persistence on treatment of single tablet regimens (STRs) with non-STR once-daily regimens in patients receiving first-line ART.
Methods: Retrospective analysis of naïve HIV-1 infected patients prospectively enrolled in the French Dat'AIDS cohort and initiating first-line ART with STRs or once-daily non-STRs from 2004 to 2013. The primary outcome was time to treatment discontinuation defined by any change in the treatment regimen. STR and non-STR groups were compared controlling for baseline risk factors by inverse probability weighted treatment Cox analysis (IPWT) and propensity-score matching (PSM).
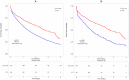
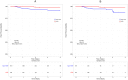
Results: Overall, 3212 patients (STR 499, non-STR 2713) were included. Median time to treatment discontinuation was shorter in non-STR patients than in STR patients, both in the IPWT (HR = 0.61, p<0.0001) and the PSM cohort (HR = 0.55, p<0.0001). This difference disappeared when censoring ART modification for simplification, both in the IPWT (HR = 0.97, p = 0.65) and the PSM cohort (HR = 0.91, p = 0.33). A lower rate of virological failure was observed with STRs than with non-STRs in both cohorts (HR = 0.23; p = 0.002 and HR = 0.22, p = 0.003, respectively). A lower rate of treatment modification for adverse event was observed with non-STRs in the IPWT cohort (HR = 1.46, p<0.0001), but not in the PSM cohort (HR = 1.22, p = 0.11).
Conclusion: First-line therapy with STRs was associated with a longer time to treatment discontinuation than with non-STRs. However, when ART modification for simplification was not considered as a failure, STRs and non-STRs were similar.
Conflict of interest statement
I have read the journal's policy and the authors of this manuscript have the following competing interests: Dr Cotte reports grants from ViiV Healthcare, MSD, non-financial support from Gilead, Janssen, BMS, ViiV Healthcare, MSD, Abbott, outside the submitted work and personal fees from Gilead, Janssen, BMS, ViiV Healthcare, MSD, Abbott. Dr Ferry has nothing to disclose. Dr Pugliese has nothing to disclose. Dr Valantin reports personal fees from ViiV Healthcare, personal fees from Janssen, other conflict of interest from BMS and MSD, outside the submitted work. Dr Allavena reports personal fees from Gilead, BMS, ViiV Healthcare, MSD and Janssen, outside the submitted work. Dr Cabié reports non-financial support from Gilead, Janssen, ViiV Healthcare, MSD, outside the submitted work. Dr Poizot-Martin reports personal fees from Gilead, personal fees from BMS, personal fees from Abbvie, personal fees from ViiV Healthcare, outside the submitted work. Dr Rey reports personal fees from Gilead, non-financial support from Gilead, MSD, and BMS, outside the submitted work. Dr Duvivier reports grants from Gilead, grants from BMS, Janssen, ViiV Healthcare, and MSD, outside the submitted work. Dr Chéret has nothing to disclose. Dr Dellamonica has nothing to disclose. Dr Parienti has nothing to disclose. Pierre Pradat performed medical writing services and received financial support from Dat’AIDS. This does not alter our adherence to PLOS ONE policies on sharing data and materials.
Figures


Similar articles
-
Real-world adherence and persistence for newly-prescribed HIV treatment: single versus multiple tablet regimen comparison among US medicaid beneficiaries.AIDS Res Ther. 2020 Apr 1;17(1):12. doi: 10.1186/s12981-020-00268-1. AIDS Res Ther. 2020. PMID: 32238169 Free PMC article.
-
Persistence and adherence to single-tablet regimens in HIV treatment: a cohort study from the French National Healthcare Insurance Database.J Antimicrob Chemother. 2015 Jul;70(7):2121-8. doi: 10.1093/jac/dkv083. Epub 2015 Apr 22. J Antimicrob Chemother. 2015. PMID: 25904729
-
Persistence to single-tablet regimen versus less-drug regimen in treatment experienced HIV-infected patients on antiretroviral therapy.Farm Hosp. 2016 Jun 1;40(4):272-8. doi: 10.7399/fh.2016.40.4.10453. Farm Hosp. 2016. PMID: 27571495 English.
-
Single-Tablet Regimens for the Treatment of HIV-1 Infection.Ann Pharmacother. 2017 Apr;51(4):332-344. doi: 10.1177/1060028016682531. Epub 2016 Nov 28. Ann Pharmacother. 2017. PMID: 27895236 Review.
-
HIV Clinical Updates: New Single-Tablet Regimens.Ann Pharmacother. 2019 Jan;53(1):82-94. doi: 10.1177/1060028018793252. Epub 2018 Aug 3. Ann Pharmacother. 2019. PMID: 30073873 Review.
Cited by
-
Cumulative tenofovir diphosphate exposure in persons with HIV taking single- vs. multiple-tablet regimens.Pharmacotherapy. 2022 Aug;42(8):641-650. doi: 10.1002/phar.2711. Epub 2022 Jun 24. Pharmacotherapy. 2022. PMID: 35707973 Free PMC article.
-
Single tablet HIV regimens facilitate virologic suppression and retention in care among treatment naïve patients.AIDS Care. 2018 Aug;30(8):1017-1024. doi: 10.1080/09540121.2018.1442554. Epub 2018 Feb 25. AIDS Care. 2018. PMID: 29478329 Free PMC article.
-
Antiretroviral switching and bedaquiline treatment of drug-resistant tuberculosis HIV co-infection.Lancet HIV. 2019 Mar;6(3):e201-e204. doi: 10.1016/S2352-3018(19)30035-9. Lancet HIV. 2019. PMID: 30846058 Free PMC article.
-
A week-48 randomized phase-3 trial of darunavir/cobicistat/emtricitabine/tenofovir alafenamide in treatment-naive HIV-1 patients.AIDS. 2018 Jul 17;32(11):1431-1442. doi: 10.1097/QAD.0000000000001817. AIDS. 2018. PMID: 29683855 Free PMC article. Clinical Trial.
-
First-line antiretroviral therapy initiation for newly diagnosed people with HIV in the Netherlands: A retrospective analysis from 2016 to 2020.PLoS One. 2024 Jul 26;19(7):e0307963. doi: 10.1371/journal.pone.0307963. eCollection 2024. PLoS One. 2024. PMID: 39058734 Free PMC article.
References
-
- European AIDS clinical society. Guidelines for the clinical management and treatment of HIV infected adults in Europe. Version 8.0, October 2015. [Internet]. [cited 31 May 2016]. http://www.eacsociety.org/files/2015_eacsguidelines_8.0-english_revised-...
-
- Claxton AJ, Cramer J, Pierce C. A systematic review of the associations between dose regimens and medication compliance. Clin Ther. 2001;23: 1296–1310. - PubMed
-
- Killingley B, Pozniak A. The first once-daily single-tablet regimen for the treatment of HIV-infected patients. Drugs Today Barc Spain 1998. 2007;43: 427–442. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

