Transmission dynamics and elimination potential of zoonotic tuberculosis in morocco
- PMID: 28152056
- PMCID: PMC5289436
- DOI: 10.1371/journal.pntd.0005214
Transmission dynamics and elimination potential of zoonotic tuberculosis in morocco
Abstract
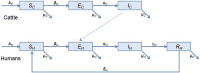
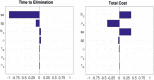
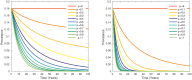
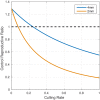
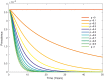
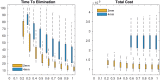
Bovine tuberculosis (BTB) is an endemic zoonosis in Morocco caused by Mycobacterium bovis, which infects many domestic animals and is transmitted to humans through consumption of raw milk or from contact with infected animals. The prevalence of BTB in Moroccan cattle is estimated at 18%, and 33% at the individual and the herd level respectively, but the human M. bovis burden needs further clarification. The current control strategy based on test and slaughter should be improved through local context adaptation taking into account a suitable compensation in order to reduce BTB prevalence in Morocco and decrease the disease burden in humans and animals. We established a simple compartmental deterministic mathematical model for BTB transmission in cattle and humans to provide a general understanding of BTB, in particular regarding transmission to humans. Differential equations were used to model the different pathways between the compartments for cattle and humans. Scenarios of test and slaughter were simulated to determine the effects of varying the proportion of tested animals (p) on the time to elimination of BTB (individual animal prevalence of less than one in a thousand) in cattle and humans. The time to freedom from disease ranged from 75 years for p = 20% to 12 years for p = 100%. For p > 60% the time to elimination was less than 20 years. The cumulated cost was largely stable: for p values higher than 40%, cost ranged from 1.47 to 1.60 billion euros with a time frame of 12 to 32 years to reach freedom from disease. The model simulations also suggest that using a 2mm cut off instead of a 4mm cut off in the Single Intradermal Comparative Cervical Tuberculin skin test (SICCT) would result in cheaper and quicker elimination programs. This analysis informs Moroccan bovine tuberculosis control policy regarding time frame, range of cost and levels of intervention. However, further research is needed to clarify the national human-bovine tuberculosis ratio in Morocco.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
Similar articles
-
Phylogenetic analysis of Mycobacterium bovis reveals animal and zoonotic tuberculosis spread between Morocco and European countries.PLoS Negl Trop Dis. 2025 Feb 18;19(2):e0011982. doi: 10.1371/journal.pntd.0011982. eCollection 2025 Feb. PLoS Negl Trop Dis. 2025. PMID: 39965004 Free PMC article.
-
Human as a potential vector of bovine tuberculosis in cattle.Ann Agric Environ Med. 2019 Sep 19;26(3):396-399. doi: 10.26444/aaem/102814. Epub 2019 Apr 10. Ann Agric Environ Med. 2019. PMID: 31559792
-
Epidemiology of Mycobacterium bovis and Mycobacterium tuberculosis in animals: Transmission dynamics and control challenges of zoonotic TB in Ethiopia.Prev Vet Med. 2018 Oct 1;158:1-17. doi: 10.1016/j.prevetmed.2018.06.012. Epub 2018 Jun 28. Prev Vet Med. 2018. PMID: 30220382 Review.
-
Bovine tuberculosis prevalence survey on cattle in the rural livestock system of Torodi (Niger).PLoS One. 2011;6(9):e24629. doi: 10.1371/journal.pone.0024629. Epub 2011 Sep 22. PLoS One. 2011. PMID: 21961039 Free PMC article.
-
Current knowledge and pending challenges in zoonosis caused by Mycobacterium bovis: a review.Res Vet Sci. 2014 Oct;97 Suppl:S94-S100. doi: 10.1016/j.rvsc.2013.11.008. Epub 2013 Dec 1. Res Vet Sci. 2014. PMID: 24360647 Review.
Cited by
-
Phylogenetic analysis of Mycobacterium bovis reveals animal and zoonotic tuberculosis spread between Morocco and European countries.PLoS Negl Trop Dis. 2025 Feb 18;19(2):e0011982. doi: 10.1371/journal.pntd.0011982. eCollection 2025 Feb. PLoS Negl Trop Dis. 2025. PMID: 39965004 Free PMC article.
-
Effectiveness and profitability of preventive veterinary interventions in controlling infectious diseases of ruminant livestock in sub-Saharan Africa: a scoping review.BMC Vet Res. 2022 Sep 2;18(1):332. doi: 10.1186/s12917-022-03428-9. BMC Vet Res. 2022. PMID: 36056387 Free PMC article.
-
Molecular characterization of bovine tuberculosis strains in two slaughterhouses in Morocco.BMC Vet Res. 2017 Aug 25;13(1):272. doi: 10.1186/s12917-017-1165-6. BMC Vet Res. 2017. PMID: 28841870 Free PMC article.
-
Multidrug-resistant strains of Mycobacterium complex species in Egyptian farm animals, veterinarians, and farm and abattoir workers.Vet World. 2020 Oct;13(10):2150-2155. doi: 10.14202/vetworld.2020.2150-2155. Epub 2020 Oct 14. Vet World. 2020. PMID: 33281349 Free PMC article.
-
Factors Affecting Herd Status for Bovine Tuberculosis in Dairy Cattle in Northern Thailand.Vet Med Int. 2017;2017:2964389. doi: 10.1155/2017/2964389. Epub 2017 May 3. Vet Med Int. 2017. PMID: 28553557 Free PMC article.
References
-
- Langer AJ, LoBue PA. Public health significance of zoonotic tuberculosis caused by the Mycobacterium tuberculosis complex Zoonotic Tuberculosis: Mycobacterium bovis and Other Pathogenic Mycobacteria. Third Edition ed2014. p. 21–33.
-
- Cosivi O, Meslin FX, Daborn CJ, Grange JM. Epidemiology of Mycobacterium bovis infection in animals and humans, with particular reference to Africa. Revue scientifique et technique. 1995;14(3):733–46. - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

