NOX1 and NOX4 are required for the differentiation of mouse F9 cells into extraembryonic endoderm
- PMID: 28152080
- PMCID: PMC5289483
- DOI: 10.1371/journal.pone.0170812
NOX1 and NOX4 are required for the differentiation of mouse F9 cells into extraembryonic endoderm
Abstract
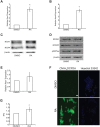
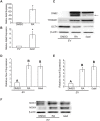
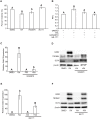
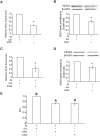
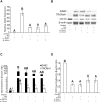
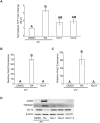
Mouse F9 cells differentiate to primitive endoderm (PrE) when treated with retinoic acid (RA). Differentiation is accompanied by increased reactive oxygen species (ROS) levels, and while treating F9 cells with antioxidants attenuates differentiation, H2O2 treatment alone is sufficient to induce PrE. We identified the NADPH oxidase (NOX) complexes as candidates for the source of this endogenous ROS, and within this gene family, and over the course of differentiation, Nox1 and Nox 4 show the greatest upregulation induced by RA. Gata6, encoding a master regulator of extraembryonic endoderm is also up-regulated by RA and we provide evidence that NOX1 and NOX4 protein levels increase in F9 cells overexpressing Gata6. Pan-NOX and NOX1-specific inhibitors significantly reduced the ability of RA to induce PrE, and this was recapitulated using a genetic approach to knockdown Nox1 and/or Nox4 transcripts. Interestingly, overexpressing either gene in untreated F9 cells did not induce differentiation, even though each elevated ROS levels. Thus, the data suggests that ROS produced during PrE differentiation is dependent in part on increased NOX1 and NOX4 levels, which is under the control of GATA6. Furthermore, these results suggest that the combined activity of multiple NOX proteins is necessary for the differentiation of F9 cells to primitive endoderm.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures

Similar articles
-
Reactive oxygen species and Wnt signalling crosstalk patterns mouse extraembryonic endoderm.Cell Signal. 2012 Dec;24(12):2337-48. doi: 10.1016/j.cellsig.2012.07.024. Epub 2012 Aug 3. Cell Signal. 2012. PMID: 22884502
-
GATA6 and FOXA2 regulate Wnt6 expression during extraembryonic endoderm formation.Stem Cells Dev. 2012 Nov 20;21(17):3220-32. doi: 10.1089/scd.2011.0492. Epub 2012 Jun 25. Stem Cells Dev. 2012. PMID: 22607194
-
Deficiency of NOX1 or NOX4 Prevents Liver Inflammation and Fibrosis in Mice through Inhibition of Hepatic Stellate Cell Activation.PLoS One. 2015 Jul 29;10(7):e0129743. doi: 10.1371/journal.pone.0129743. eCollection 2015. PLoS One. 2015. PMID: 26222337 Free PMC article.
-
Combating oxidative stress in vascular disease: NADPH oxidases as therapeutic targets.Nat Rev Drug Discov. 2011 Jun;10(6):453-71. doi: 10.1038/nrd3403. Nat Rev Drug Discov. 2011. PMID: 21629295 Free PMC article. Review.
-
Unchanged NADPH Oxidase Activity in Nox1-Nox2-Nox4 Triple Knockout Mice: What Do NADPH-Stimulated Chemiluminescence Assays Really Detect?Antioxid Redox Signal. 2016 Mar 1;24(7):392-9. doi: 10.1089/ars.2015.6314. Epub 2015 Jun 30. Antioxid Redox Signal. 2016. PMID: 25906178 Review.
Cited by
-
Metabolic profile and differentiation potential of extraembryonic endoderm-like cells.Cell Death Discov. 2018 Sep 26;4:42. doi: 10.1038/s41420-018-0102-1. eCollection 2018. Cell Death Discov. 2018. PMID: 30302276 Free PMC article.
-
O-GlcNAcylation and Regulation of Galectin-3 in Extraembryonic Endoderm Differentiation.Biomolecules. 2022 Apr 22;12(5):623. doi: 10.3390/biom12050623. Biomolecules. 2022. PMID: 35625551 Free PMC article.
-
Parp3 promotes astrocytic differentiation through a tight regulation of Nox4-induced ROS and mTorc2 activation.Cell Death Dis. 2020 Nov 6;11(11):954. doi: 10.1038/s41419-020-03167-5. Cell Death Dis. 2020. PMID: 33159039 Free PMC article.
-
Emerging Evidence of the Significance of Thioredoxin-1 in Hematopoietic Stem Cell Aging.Antioxidants (Basel). 2022 Jun 29;11(7):1291. doi: 10.3390/antiox11071291. Antioxidants (Basel). 2022. PMID: 35883782 Free PMC article. Review.
-
Monoamine oxidase A-dependent ROS formation modulates human cardiomyocyte differentiation through AKT and WNT activation.Basic Res Cardiol. 2023 Jan 20;118(1):4. doi: 10.1007/s00395-023-00977-4. Basic Res Cardiol. 2023. PMID: 36670288 Free PMC article.
References
-
- Strickland S, Smith KK, Marotti KR. Hormonal induction of differentiation in teratocarcinoma stem cells: generation of parietal endoderm by retinoic acid and dibutyryl cAMP. Cell. 1980;21(2):347–55. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

