Safety and immunogenicity of GamEvac-Combi, a heterologous VSV- and Ad5-vectored Ebola vaccine: An open phase I/II trial in healthy adults in Russia
- PMID: 28152326
- PMCID: PMC5360131
- DOI: 10.1080/21645515.2016.1238535
Safety and immunogenicity of GamEvac-Combi, a heterologous VSV- and Ad5-vectored Ebola vaccine: An open phase I/II trial in healthy adults in Russia
Abstract
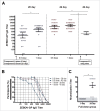
Ebola hemorrhagic fever, also known as Ebola virus disease or EVD, is one of the most dangerous viral diseases in humans and animals. In this open-label, dose-escalation clinical trial, we assessed the safety, side effects, and immunogenicity of a novel, heterologous prime-boost vaccine against Ebola, which was administered in 2 doses to 84 healthy adults of both sexes between 18 and 55 years. The vaccine consists of live-attenuated recombinant vesicular stomatitis virus (VSV) and adenovirus serotype-5 (Ad5) expressing Ebola envelope glycoprotein. The most common adverse event was pain at the injection site, although no serious adverse events were reported. The vaccine did not significantly impact blood, urine, and immune indices. Seroconversion rate was 100 %. Antigen-specific IgG geometric mean titer at day 42 was 3,277 (95 % confidence interval 2,401-4,473) in volunteers immunized at full dose. Neutralizing antibodies were detected in 93.1 % of volunteers immunized at full dose, with geometric mean titer 20. Antigen-specific response in peripheral blood mononuclear cells was also detected in 100 % of participants, as well as in CD4+ and CD8+ T cells in 82.8 % and 58.6 % of participants vaccinated at full dose, respectively. The data indicate that the vaccine is safe and induces strong humoral and cellular immune response in up to 100 % of healthy adult volunteers, and provide a rationale for testing efficacy in Phase III trials. Indeed, the strong immune response to the vaccine may elicit long-term protection. This trial was registered with grls.rosminzdrav.ru (No. 495*), and with zakupki.gov.ru (No. 0373100043215000055).
Keywords: Ad5; Ebola; Ebola virus disease; VSV; ZEBOV; heterologous vaccination; prime-boost; vaccine.
Figures


References
-
- Sanchez A, Geisbert TW, Feldmann H. Filoviridae: Marburg and Ebola viruses In: Knipe DM, Howley PM, Griffin DE, Lamb RA, Martin MA, Roizman B, Straus SE, editors Fields virology fifth edition volume 1. Philadelphia (PA: ): Lippincott Williams & Wilkins; 2007, p. 1409-48.
-
- Taylor DJ, Leach RW, Bruenn J. Filoviruses are ancient and integrated into mammalian genomes. BMC Evol Biol 2010; 10:193; PMID:20569424; http://dx.doi.org/10.1186/1471-2148-10-193 - DOI - PMC - PubMed
-
- Geisbert TW. Marburg and Ebola hemorrhagic fever (Filoviruses) In: Bennet JE, Dolin R, Blaser MJ, editors Mandell, Douglas, and Bennett's principles and practice of infectious diseases eighth edition. Philadelphia (PA: ): Elsevier; 2015, p 1995-9.
-
- World Health Organization Ebola situation report-21 October 2015. Geneva (Switzerland: ): World Health Organization; 2015 Oct 21 http://apps.who.int/ebola/current-situation/ebola-situation-report-21-oc...
-
- Sridhar S. Clinical development of Ebola vaccines. Ther Adv Vaccines 2015; 3(5–6): 125-38; PMID:26668751; http://dx.doi.org/10.1177/2051013615611017 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
